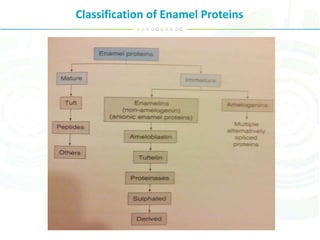
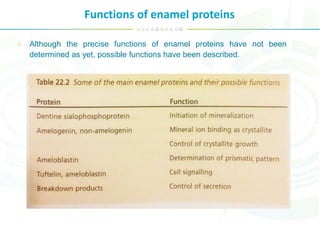
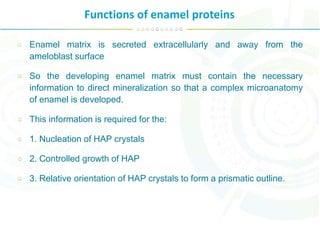
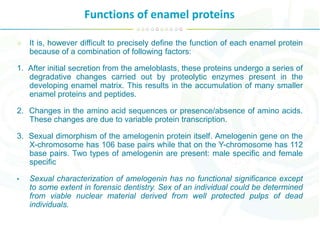
The document summarizes enamel proteins, which make up 25-30% of the early enamel matrix but only 1% of mature enamel. It describes how amelogenins, which comprise 90% of immature enamel, are degraded during maturation. Amelogenins form nanospheres that regulate mineralization and crystal growth. Non-amelogenin proteins include enamelins, ameloblastin, tuftelin, and others. While protein functions are not fully known, they are involved in nucleation, orientation and growth of hydroxyapatite crystals during enamel development.