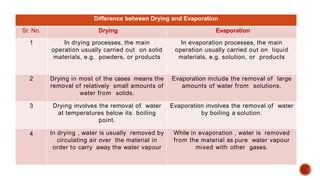
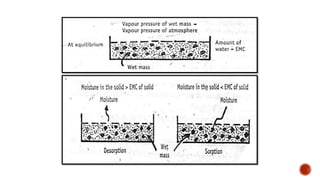
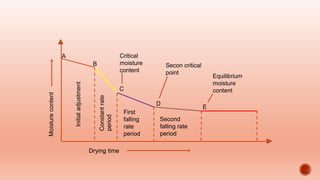
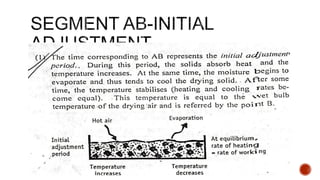
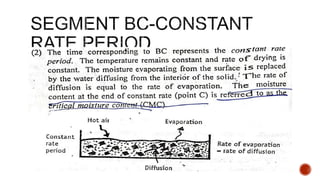
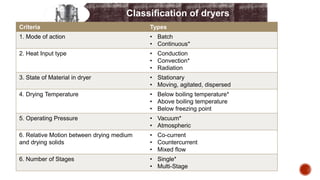
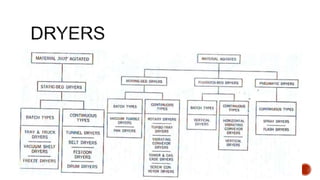
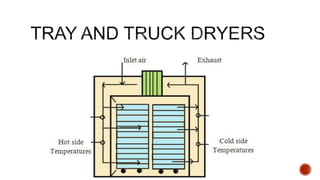
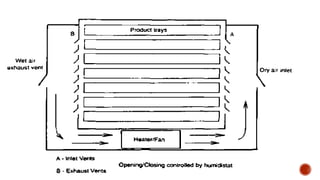
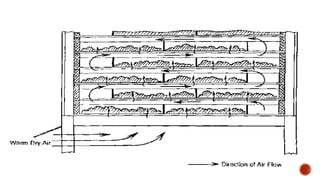
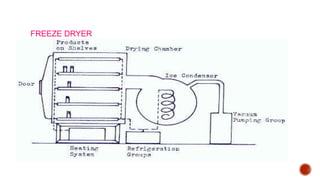
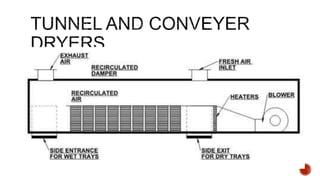
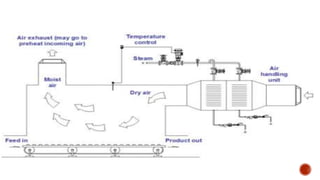
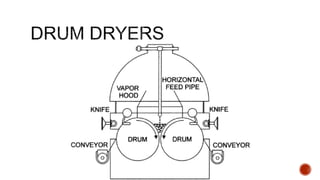
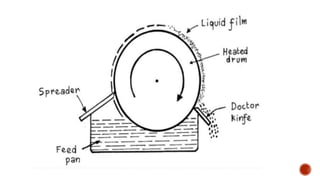
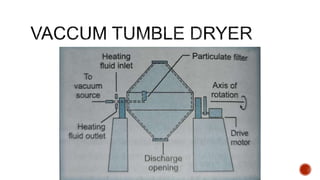
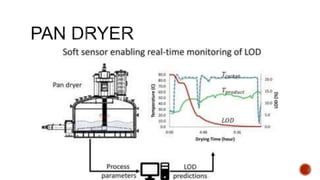
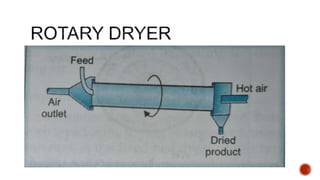
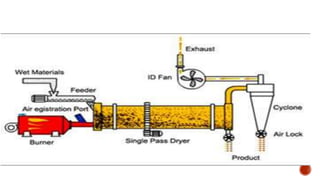
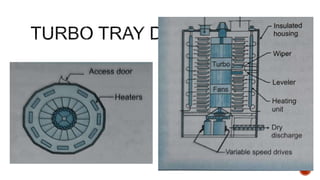
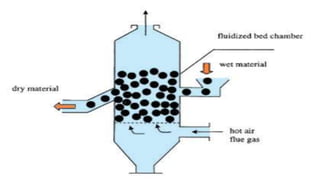
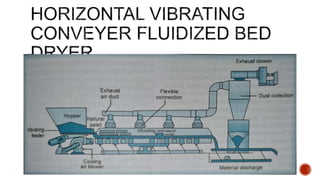
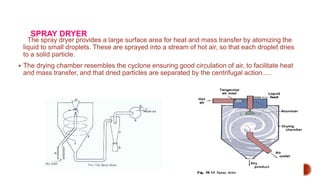
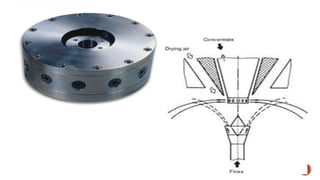
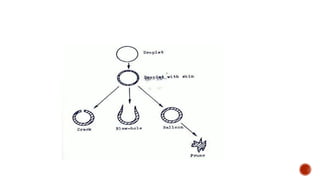
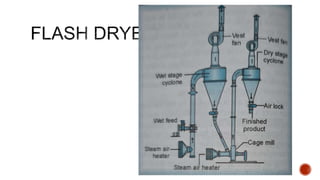
The document discusses various non-thermal drying processes, detailing the fundamental differences between drying and evaporation, along with their applications in pharmaceuticals. It explains the mechanisms of drying, the importance of controlling moisture levels, and different parameters that affect drying efficiency, such as material properties and processing conditions. Additionally, it outlines drying technologies like freeze-drying, their advantages, limits, and specific uses in preserving heat-sensitive materials.