Embed presentation
Download to read offline

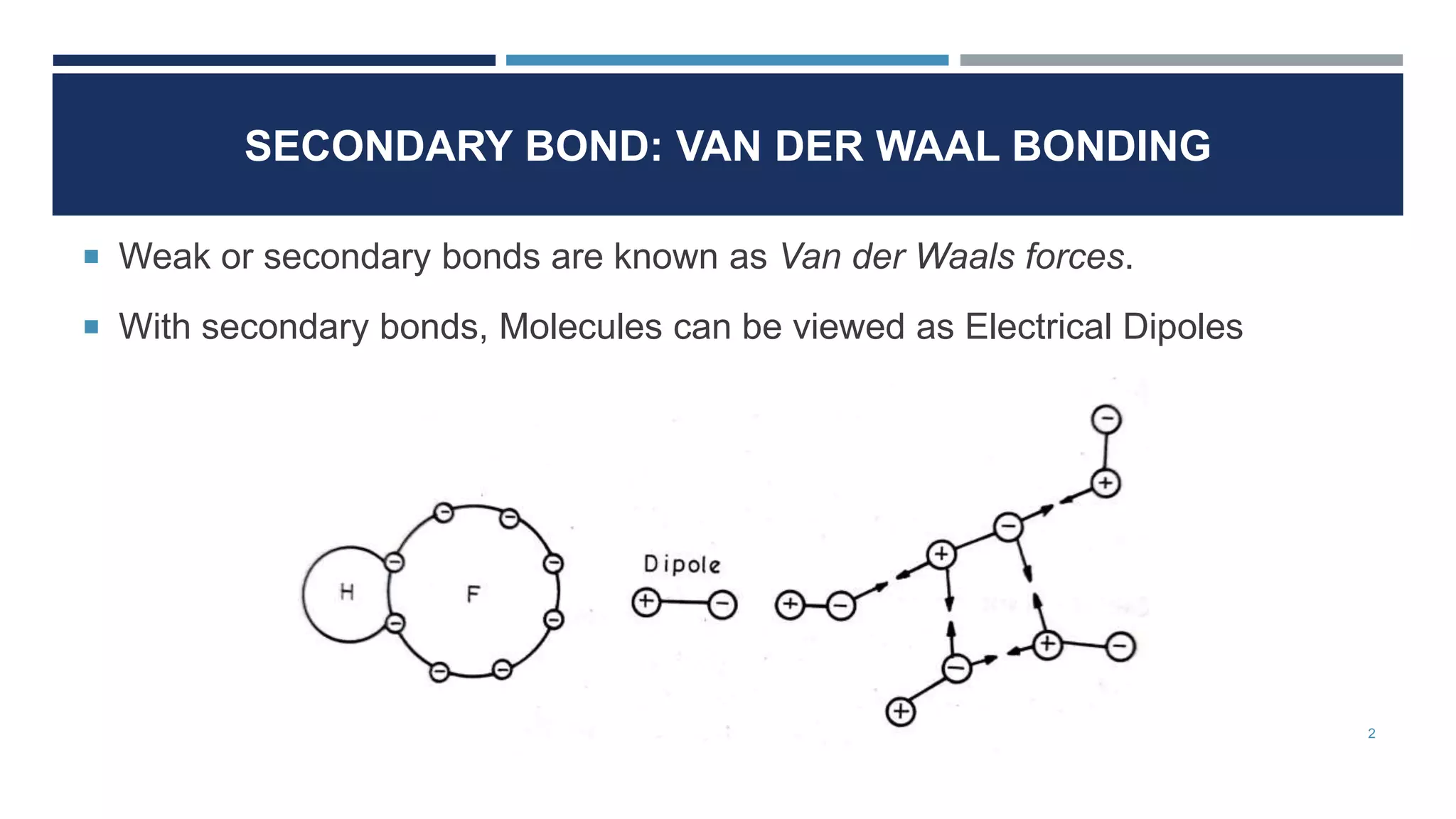
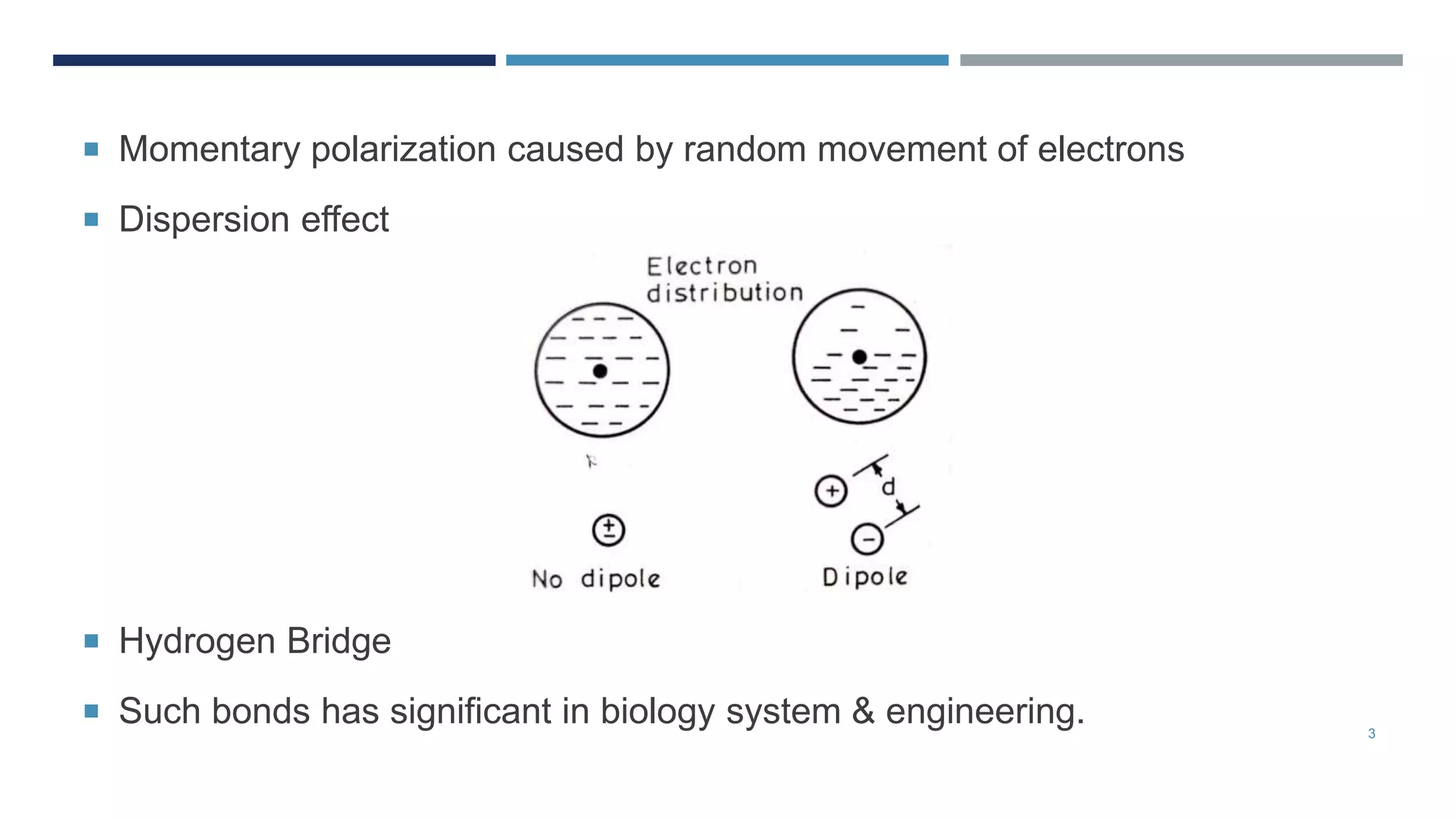
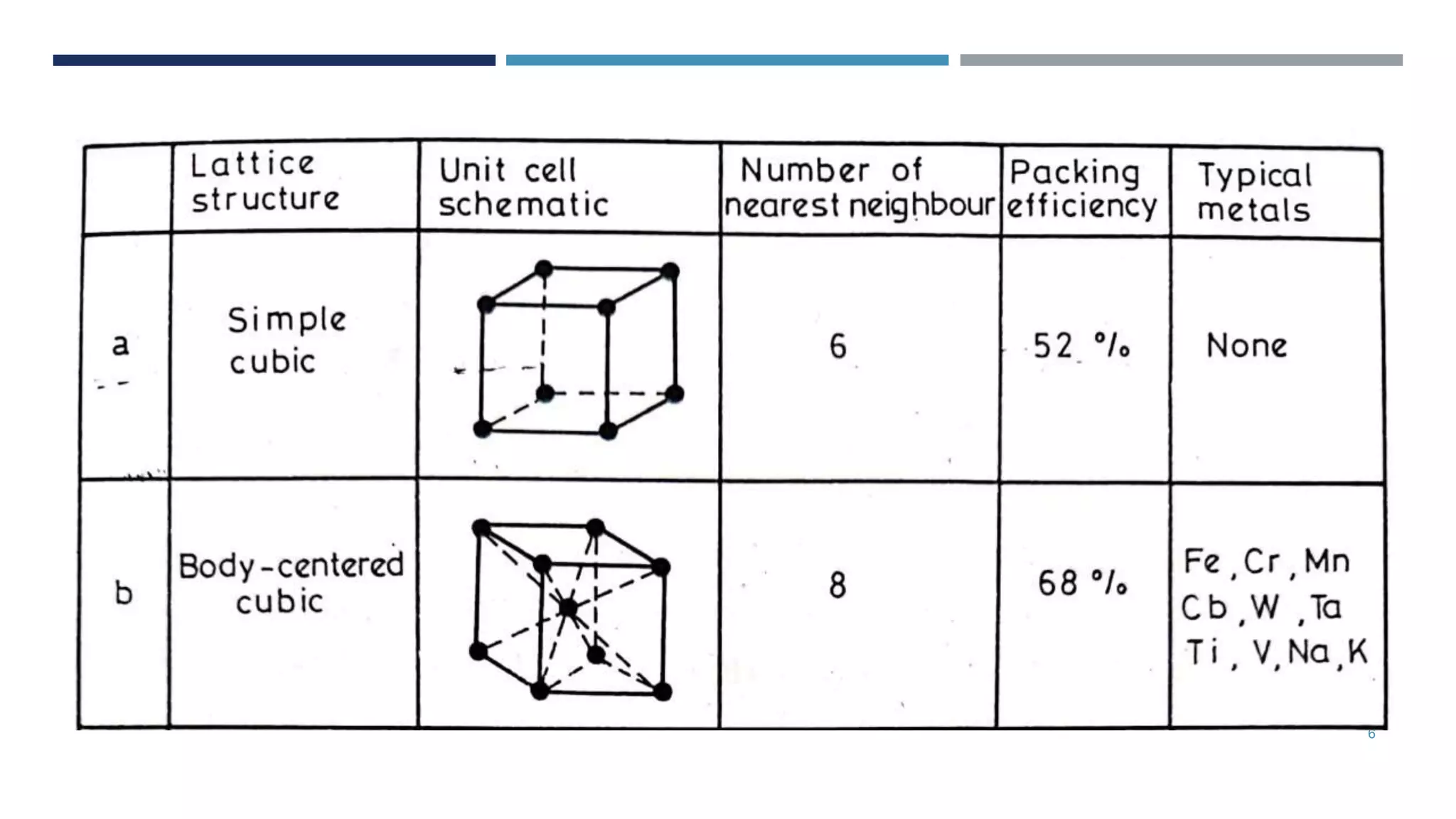
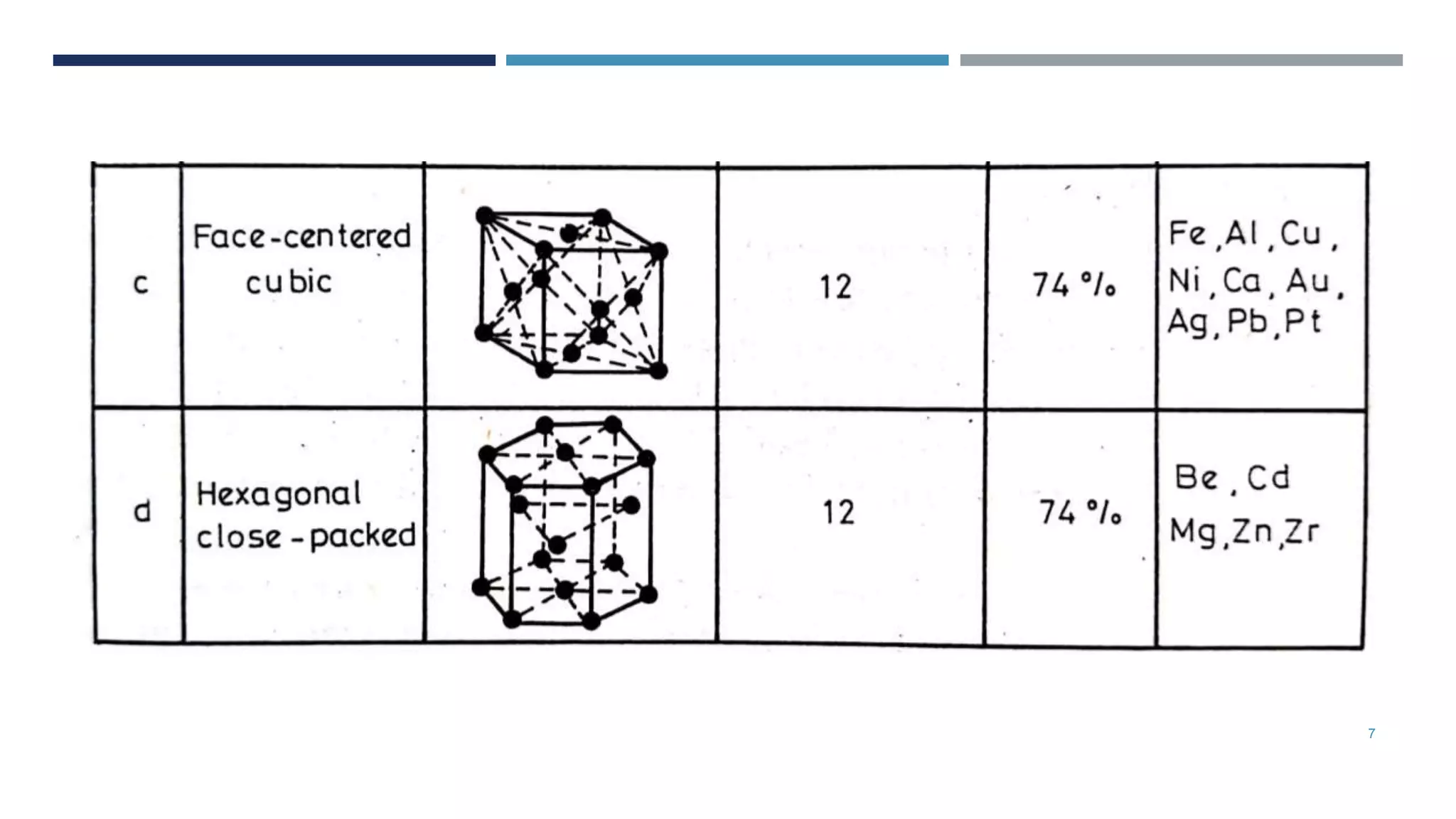

Van der Waals forces are weak secondary bonds that occur between molecules due to momentary polarization caused by electron movement, dispersion effects, and hydrogen bridging. The arrangement of atoms in a material, whether in molecular, crystalline, or amorphous structures, has a significant effect on its properties. Metals have metallic bonding and solidify into lattice structures when cooled, with many metals exhibiting allotropic changes in crystal structure.







