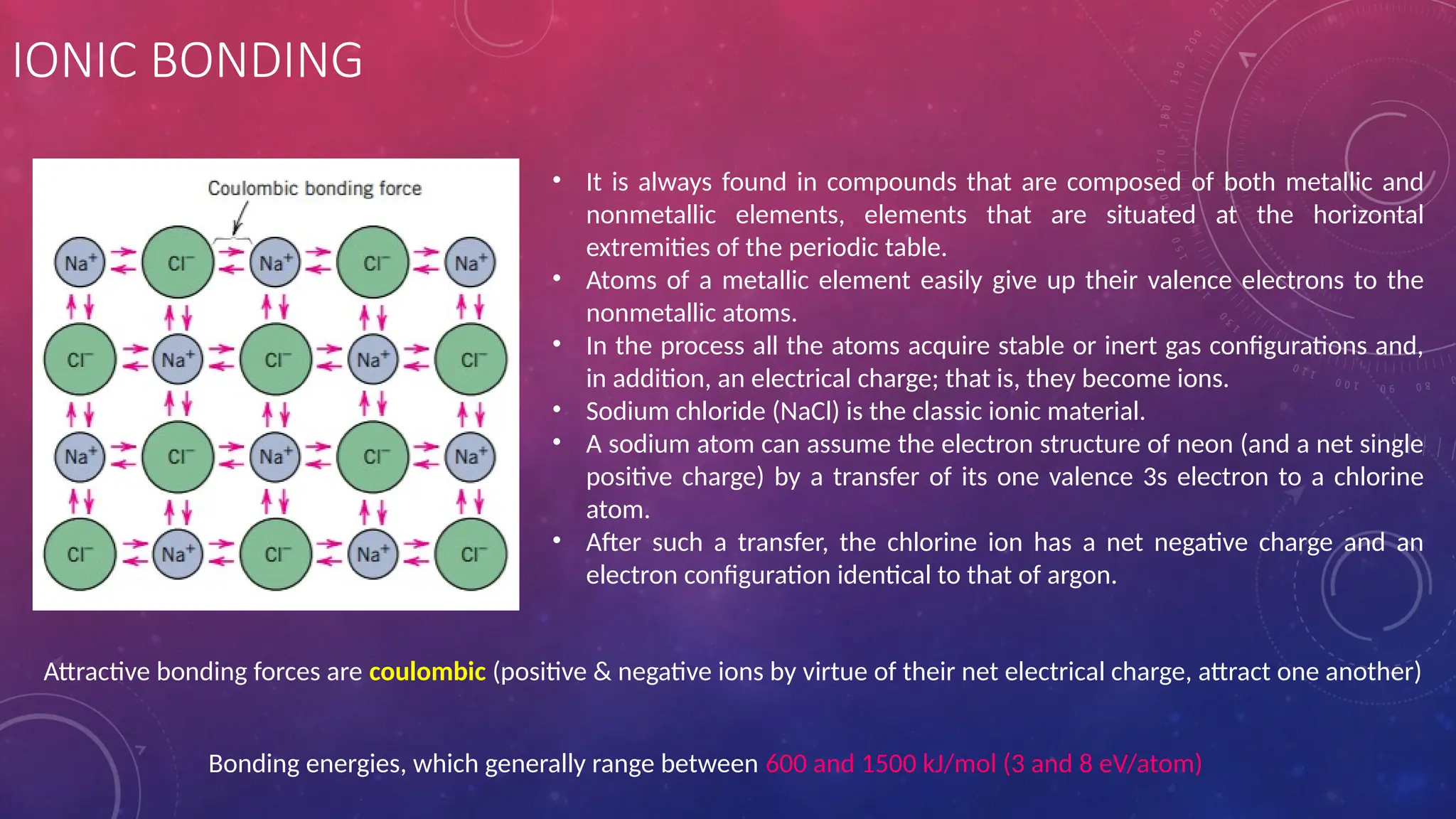
The document covers an introduction to materials science and engineering, focusing on atomic structure, interatomic bonding, and the classification of engineering materials such as metals, ceramics, and polymers. It explains the relationship between the structure, properties, processing, and performance of materials, along with detailed descriptions of atomic models, quantum numbers, bonding types (ionic, covalent, metallic), and various material classifications. Additionally, it discusses specific characteristics and applications of different metals and alloys.









![ELECTRON CONFIGURATION - EXAMPLE
First, the valence electrons are those that occupy the outermost shell.
In addition, some atoms have what are termed stable electron configurations; that
is, the states within the outermost or valence electron shell are completely filled.
Kr [36] 1s2
2s2
2p6
3s2
3p6
4s2
3d10
4p6](https://image.slidesharecdn.com/introductiontomaterialsscienceandengineering-241127134310-be42d752/75/Introduction-to-Materials-Science-and-Engineering-pptx-10-2048.jpg)