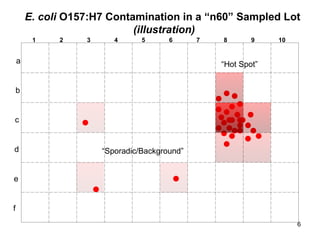
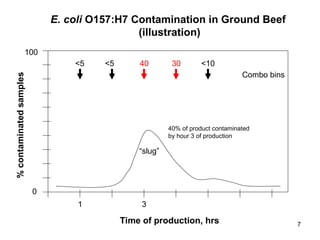
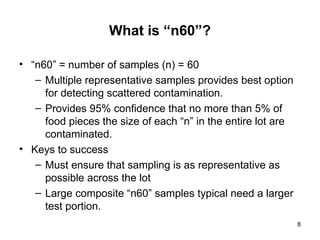
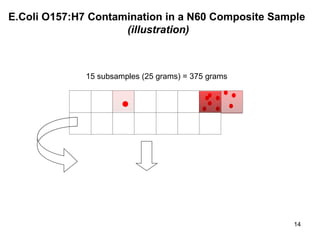
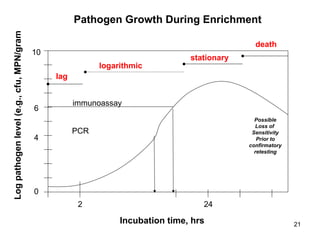
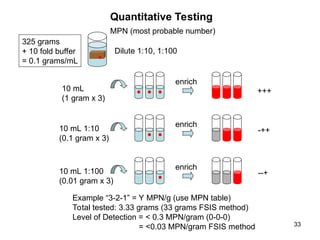
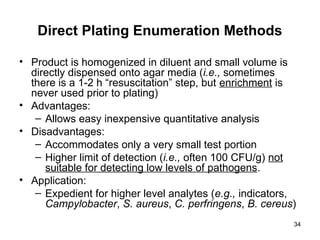
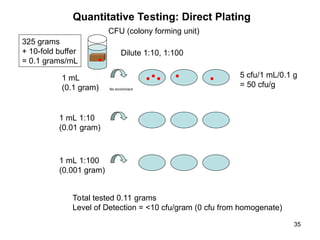
The document outlines the microbiological sampling and testing procedures essential for food safety programs, emphasizing the significance of bacteria testing in preventing foodborne illnesses. It discusses various sampling methods, laboratory testing protocols, and the importance of representative test portions for accurate detection of pathogens like E. coli O157:H7. Additionally, the document highlights challenges in pathogen detection and the need for rigorous validation of testing methods to ensure compliance with FSIS guidelines.