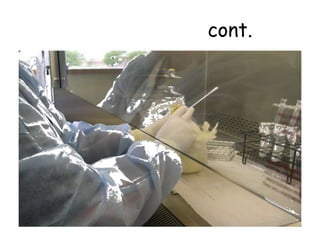
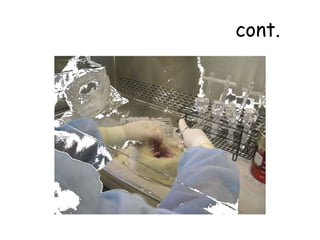
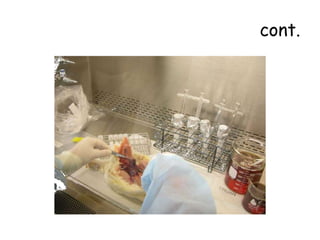
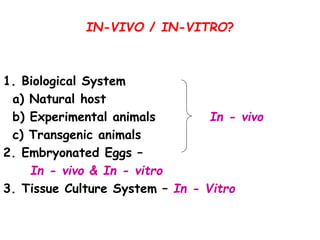
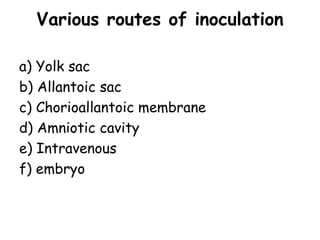
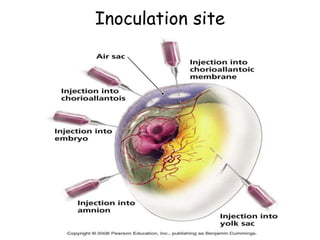
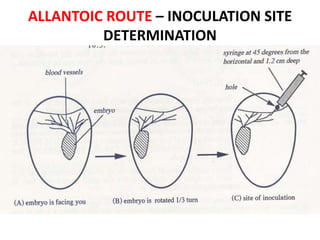
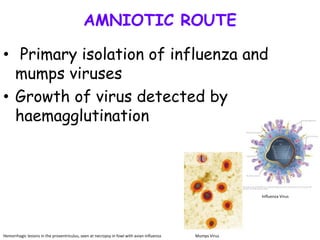
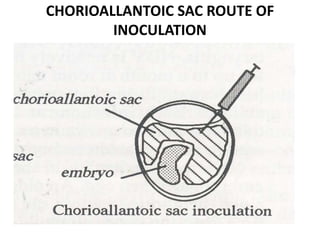
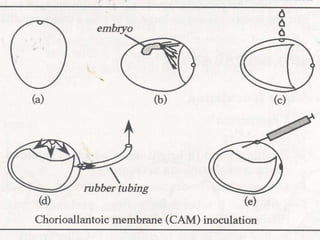
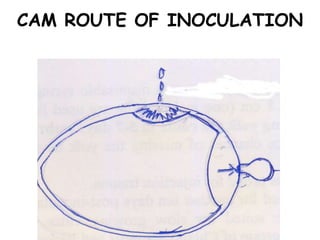
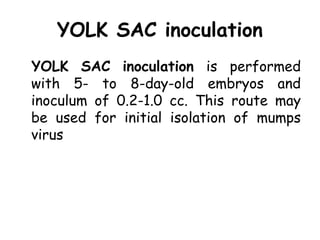
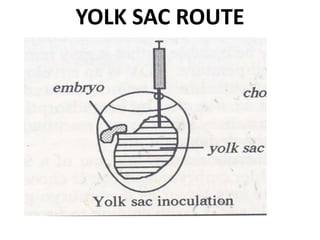
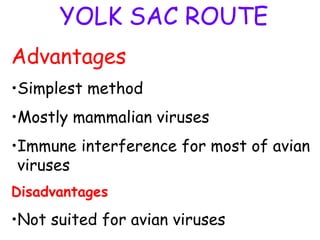
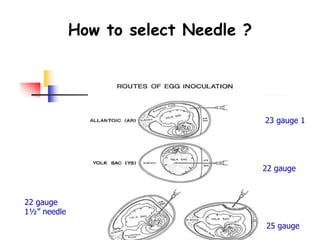
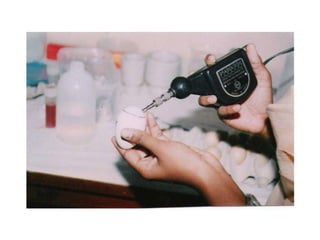
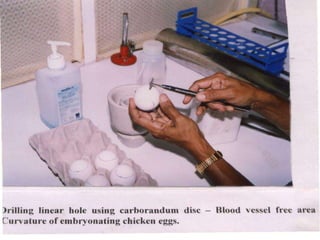
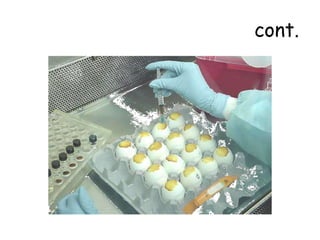
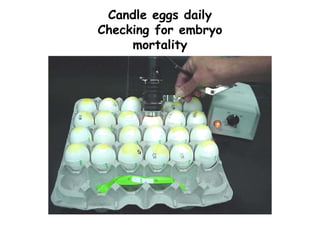
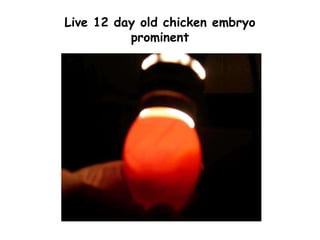
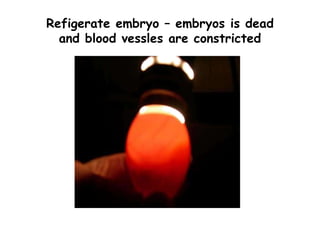
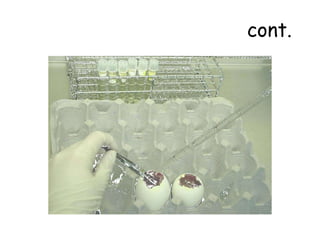
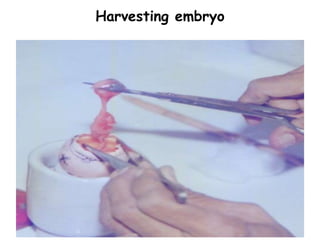
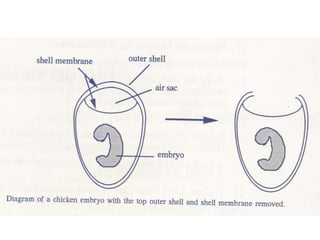
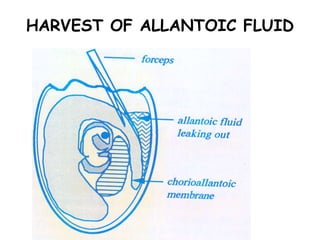
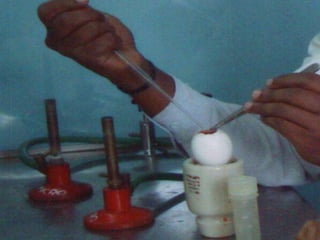
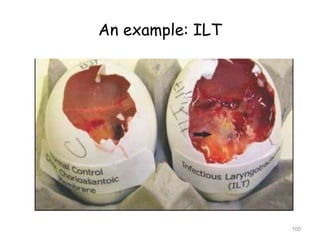
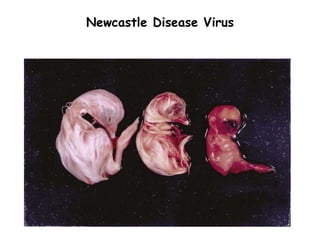
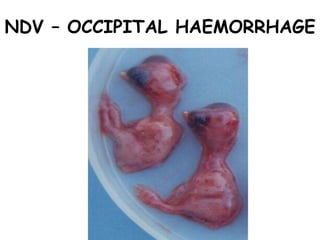
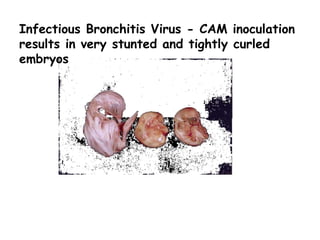
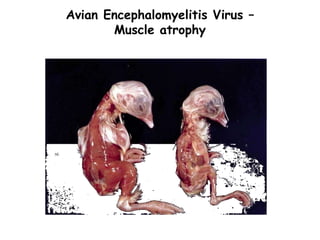
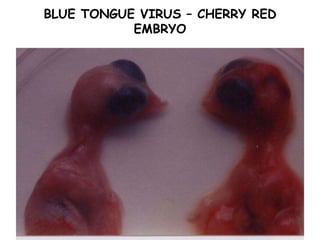
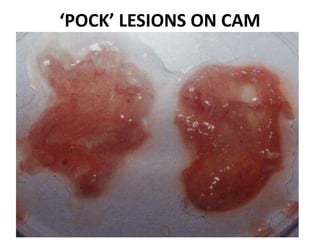
The document provides guidelines for virus isolation techniques using embryonated eggs, outlining various inoculation routes including allantoic, amniotic, chorioallantoic membrane and discussing advantages of different routes for isolating specific viruses. It describes the process of candling eggs to check embryo viability and mark inoculation sites prior to introducing viral samples via needle injection into selected areas. Proper egg storage, cleaning, and incubator maintenance are also covered.