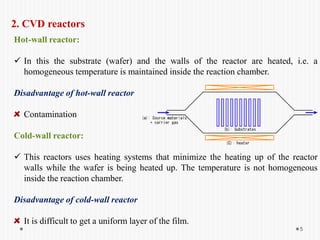
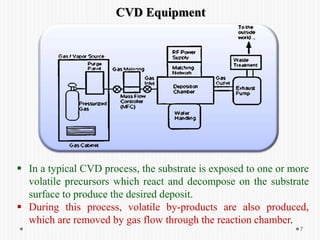
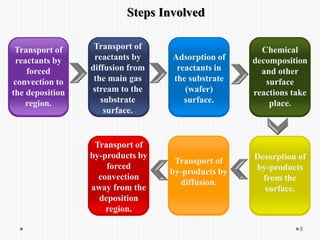
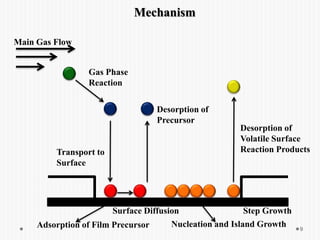
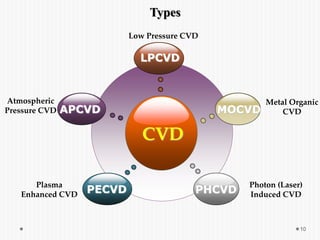
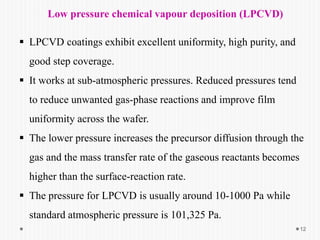
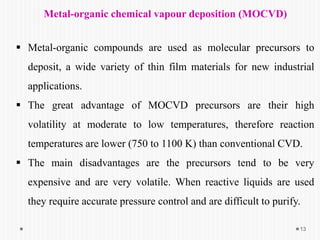
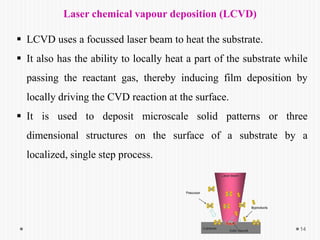
Chemical vapor deposition (CVD) involves depositing a solid material onto a substrate through chemical reactions of vapor phase precursors. CVD systems include precursor supply, heated reactors to decompose precursors, and effluent gas handling. In CVD, precursors are transported to the substrate surface through diffusion and convection, react on the surface, and deposit the solid material as a thin film while volatile byproducts desorb. CVD is used to deposit a variety of materials for applications in semiconductors, coatings, fiber optics, and microelectronics.