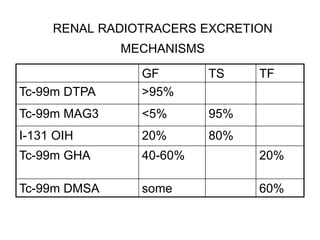
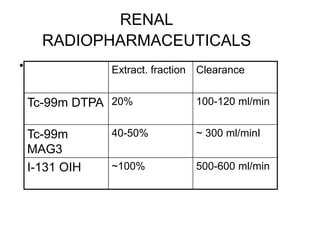
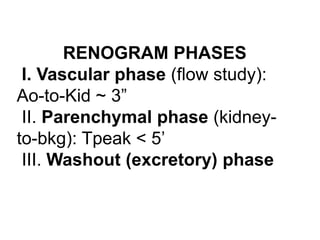
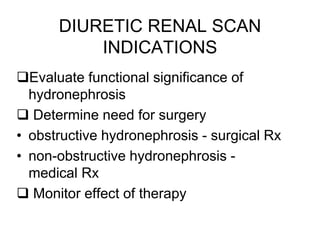
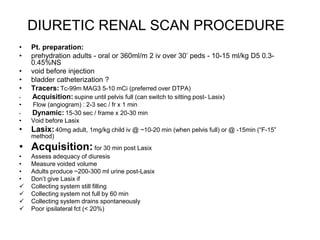
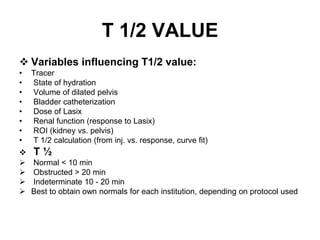
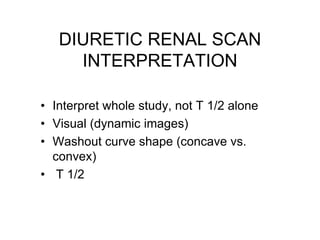
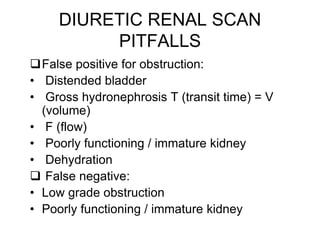
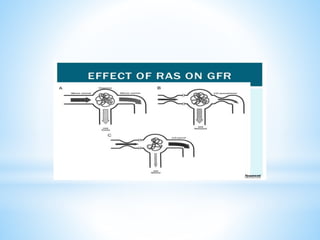
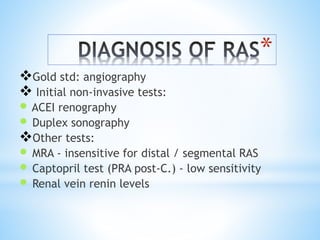
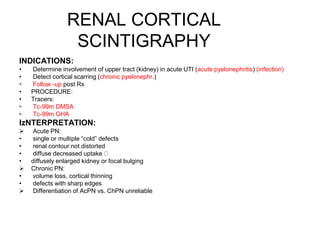
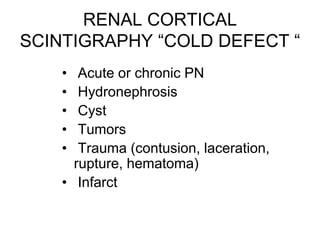
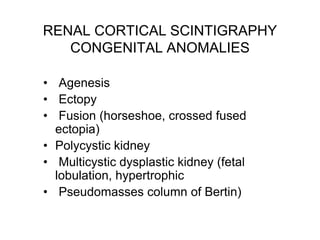
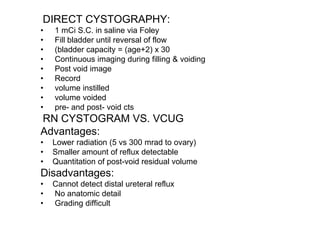
Renal scintigraphy can be used to evaluate renal infection in several ways. It can detect acute renal involvement in a urinary tract infection using tracers like Tc-99m DMSA that identify areas of reduced renal uptake, indicating infection. It can also detect cortical scarring from a prior or chronic infection. Following treatment, renal scintigraphy can monitor for resolution of the infection or scarring. The test provides important clinical information about renal involvement that cannot be reliably determined through other means. It allows for early diagnosis and treatment of infection or scarring to prevent long-term kidney damage.