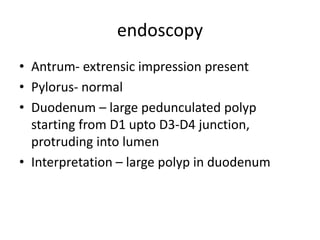
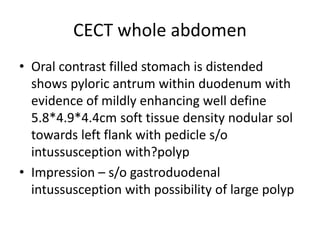
This document describes a case of a 32-year-old female patient presenting with abdominal pain, nausea, bloating and vomiting for 1 year. An endoscopy revealed a large pedunculated polyp in the duodenum, and imaging showed duodenal intussusception likely caused by the polyp. The patient underwent surgery to remove the 5x5x4cm polyp and had a feeding jejunostomy placed. Histopathology of the polyp found tubulovillous adenoma with low-grade dysplasia. Duodenal polyps are often asymptomatic but can cause bleeding or obstruction. Treatment involves endoscopic or surgical polypectomy depending on the size and location of the polyp.