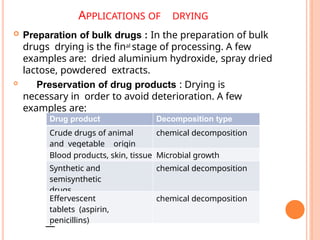
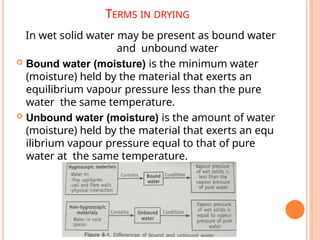
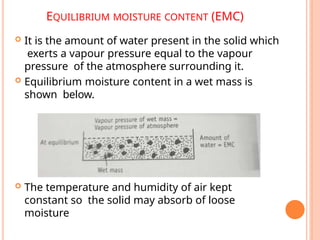
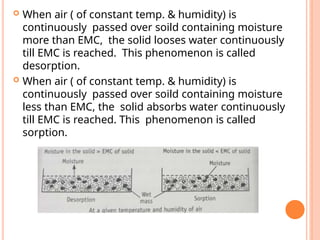
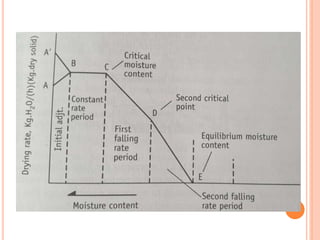
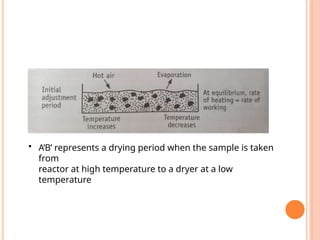
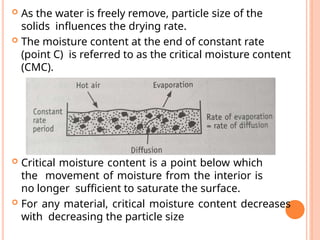
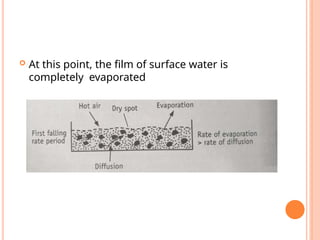
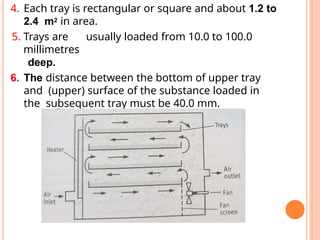
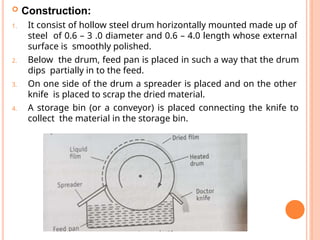
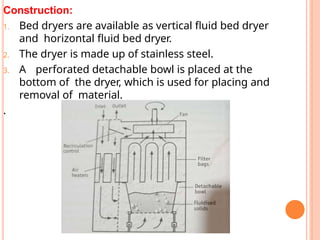
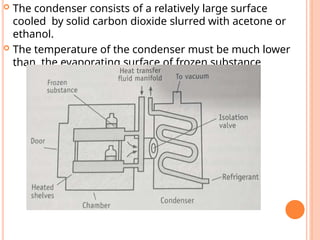
The document provides an extensive overview of drying processes, emphasizing its importance in pharmaceutical applications, including drug preservation and the enhancement of product characteristics. It discusses different drying mechanisms, theories, and equipment such as tray driers, drum driers, and spray driers, detailing their principles, construction, advantages, and disadvantages. The document also covers concepts like equilibrium moisture content, measurement techniques, and factors affecting moisture content during drying.