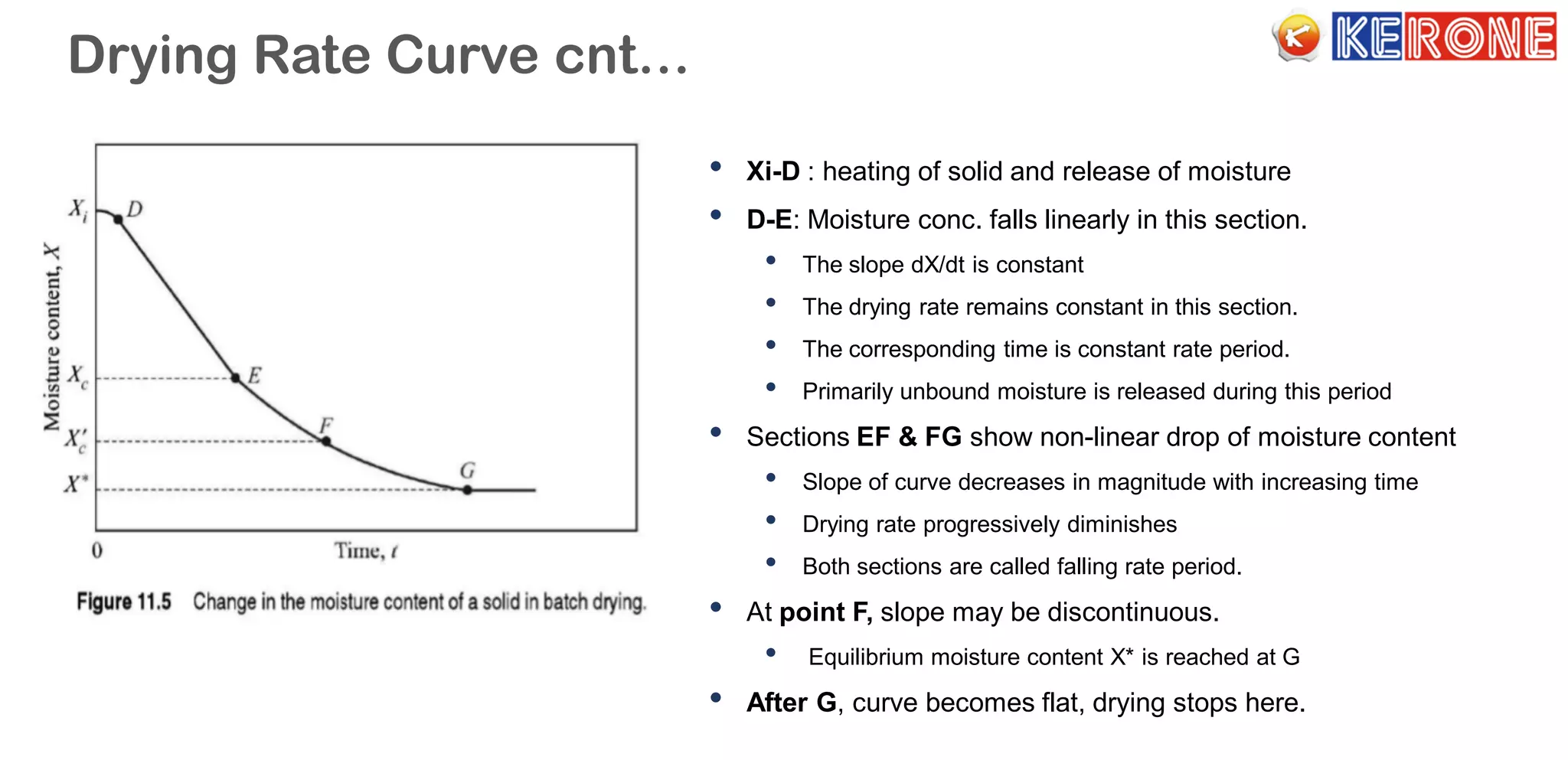
The document provides an overview of a company specializing in engineering solutions for the drying of wet solids, with over 40 years of expertise and various quality certifications. It explains the drying process, including mechanisms of moisture transport and different types of dryers used in industries. The document also covers the principles of drying, drying rate curves, and methods for calculating drying time, as well as critical moisture content and various classifications of dryer systems.