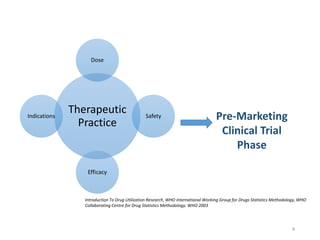
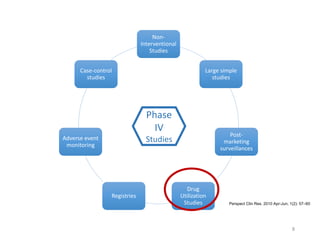
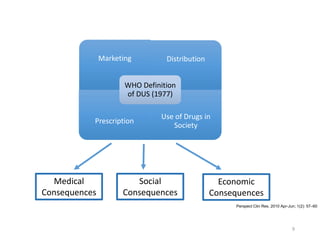
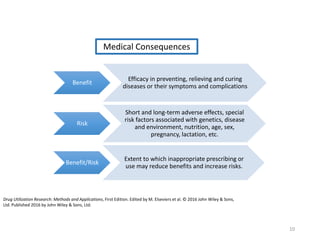
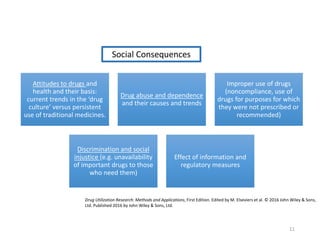
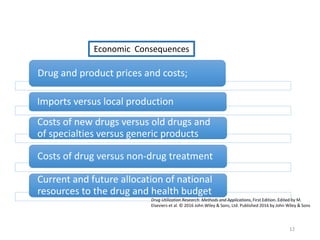
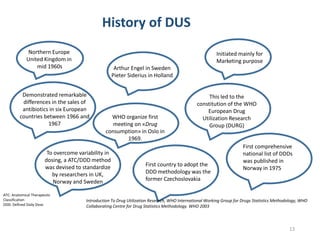
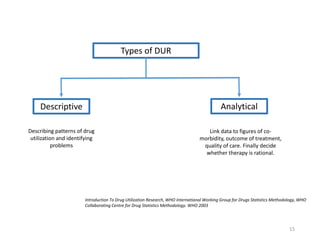
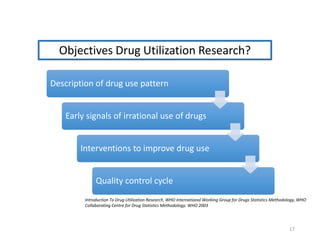
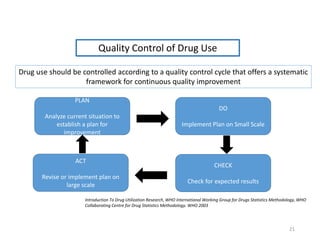
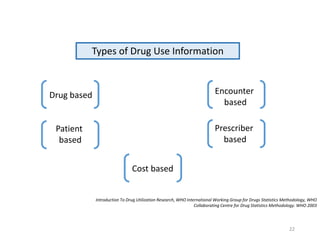
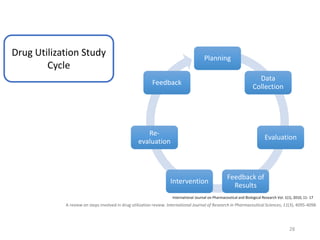
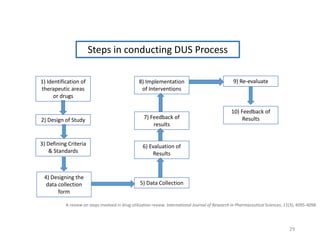
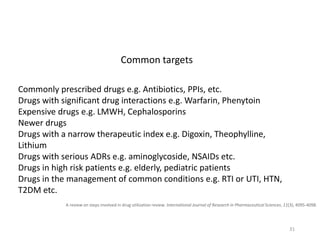
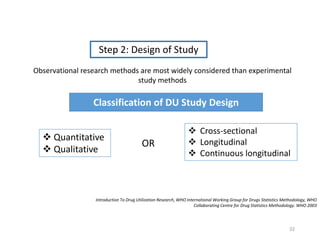
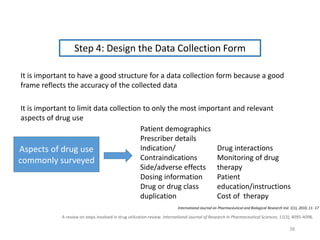
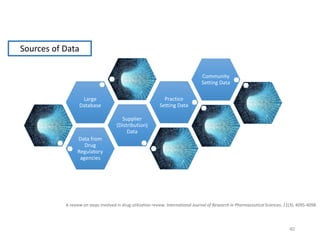
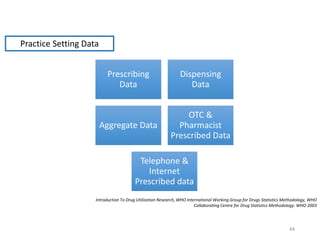
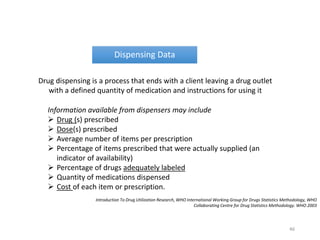
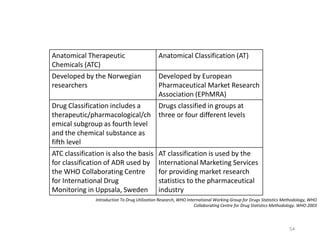
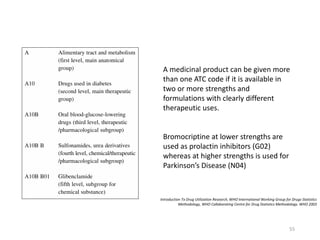
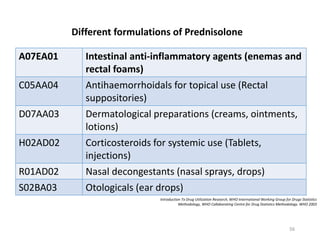
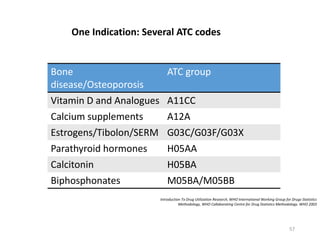
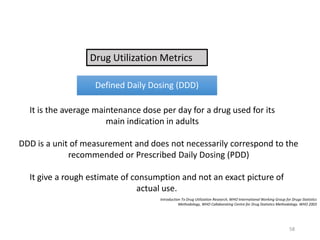
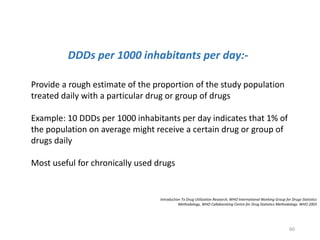
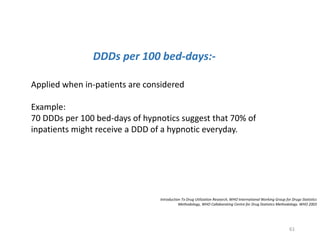
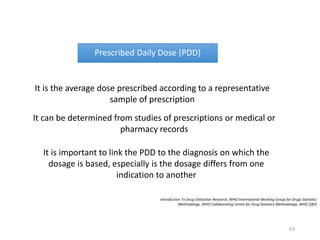
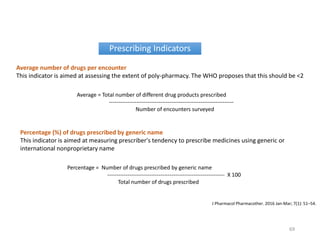
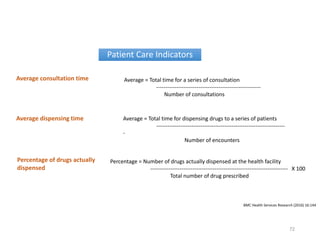
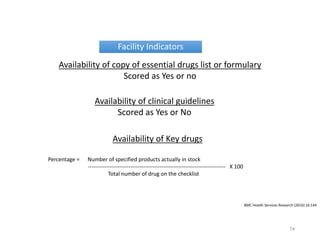
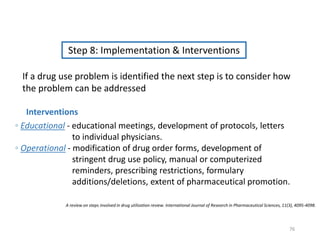
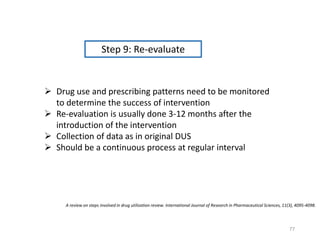
The document discusses drug utilization studies (DUS) and their significance in understanding drug use patterns and improving therapeutic practices. It outlines the steps involved in conducting DUS, limitations of pre-marketing clinical trials, and emphasizes the need for post-marketing evaluations to ensure drug safety and efficacy. The document also details various types of drug utilization information and explores the implications of drug use on medical, social, and economic factors.