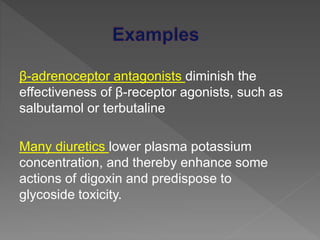
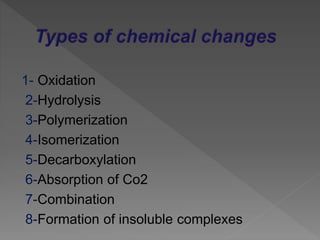
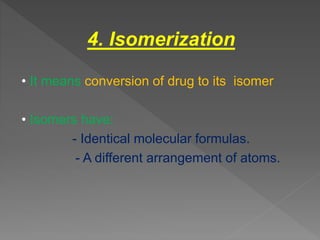
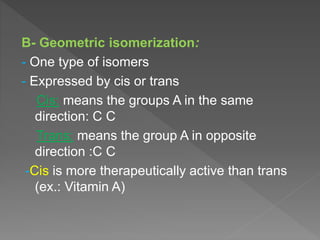
Drug incompatibility refers to interactions between substances in a pharmaceutical dosage form that can change its chemical, physical, or therapeutic properties. There are three main types: therapeutic (or drug interactions), physical, and chemical incompatibilities. Therapeutic incompatibilities involve effects on absorption, distribution, metabolism, or excretion of one drug by another drug. Common mechanisms are altered gastrointestinal absorption, plasma protein binding displacement, effects on metabolism by the liver or other organs, and altered renal excretion. Chemical incompatibilities can occur when substances precipitate, undergo hydrolysis, oxidation, polymerization, or other chemical reactions on mixing. Proper screening is needed to avoid incompatibilities when formulating drug combinations.