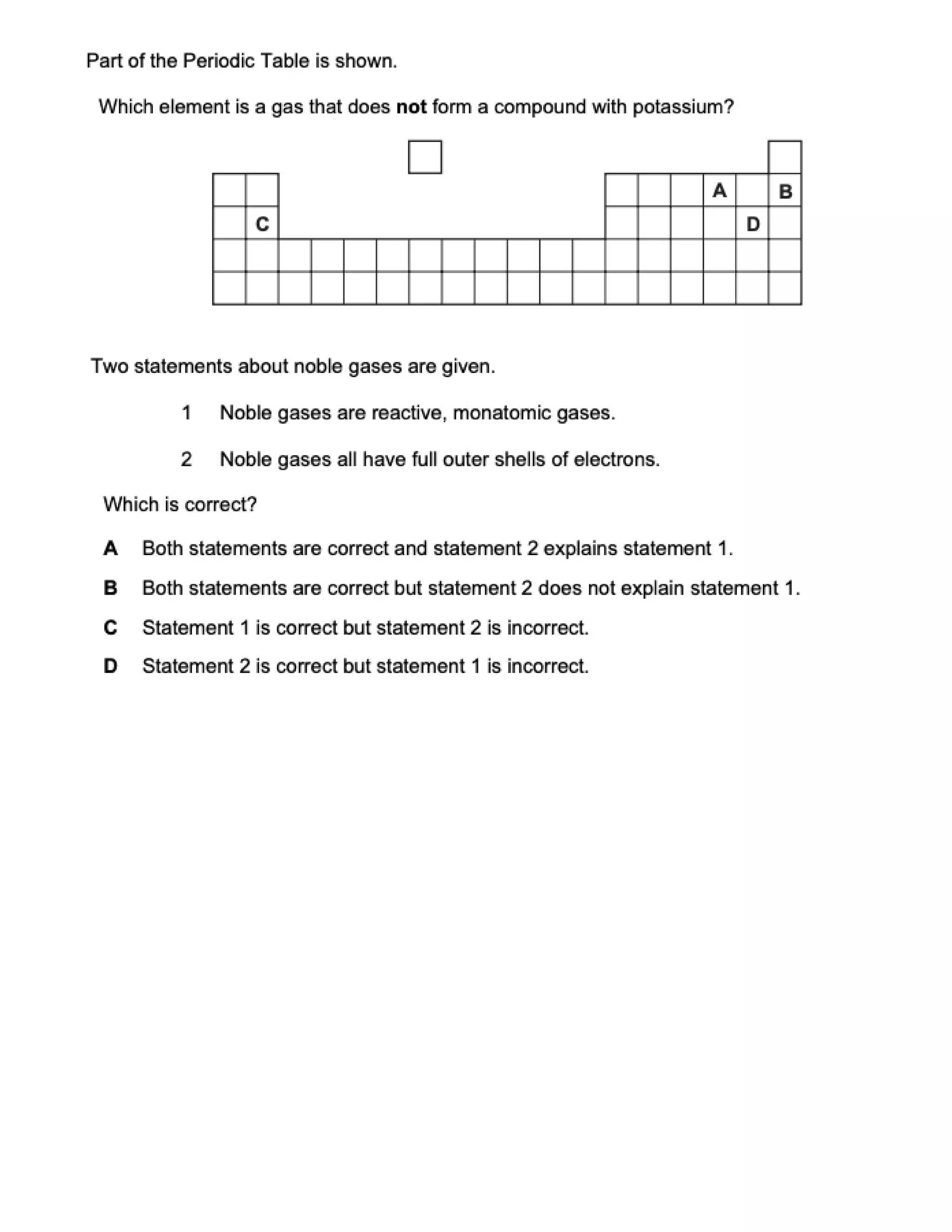
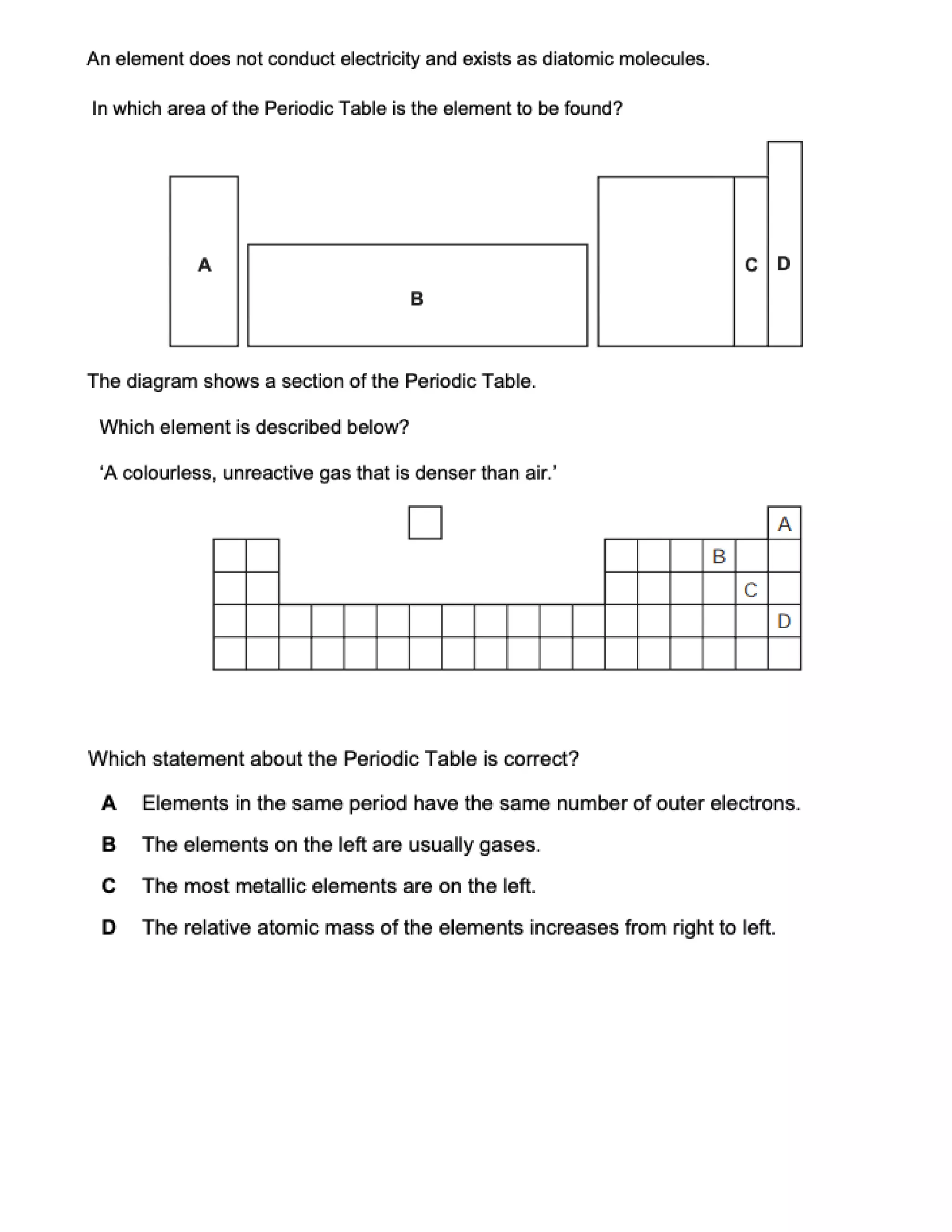
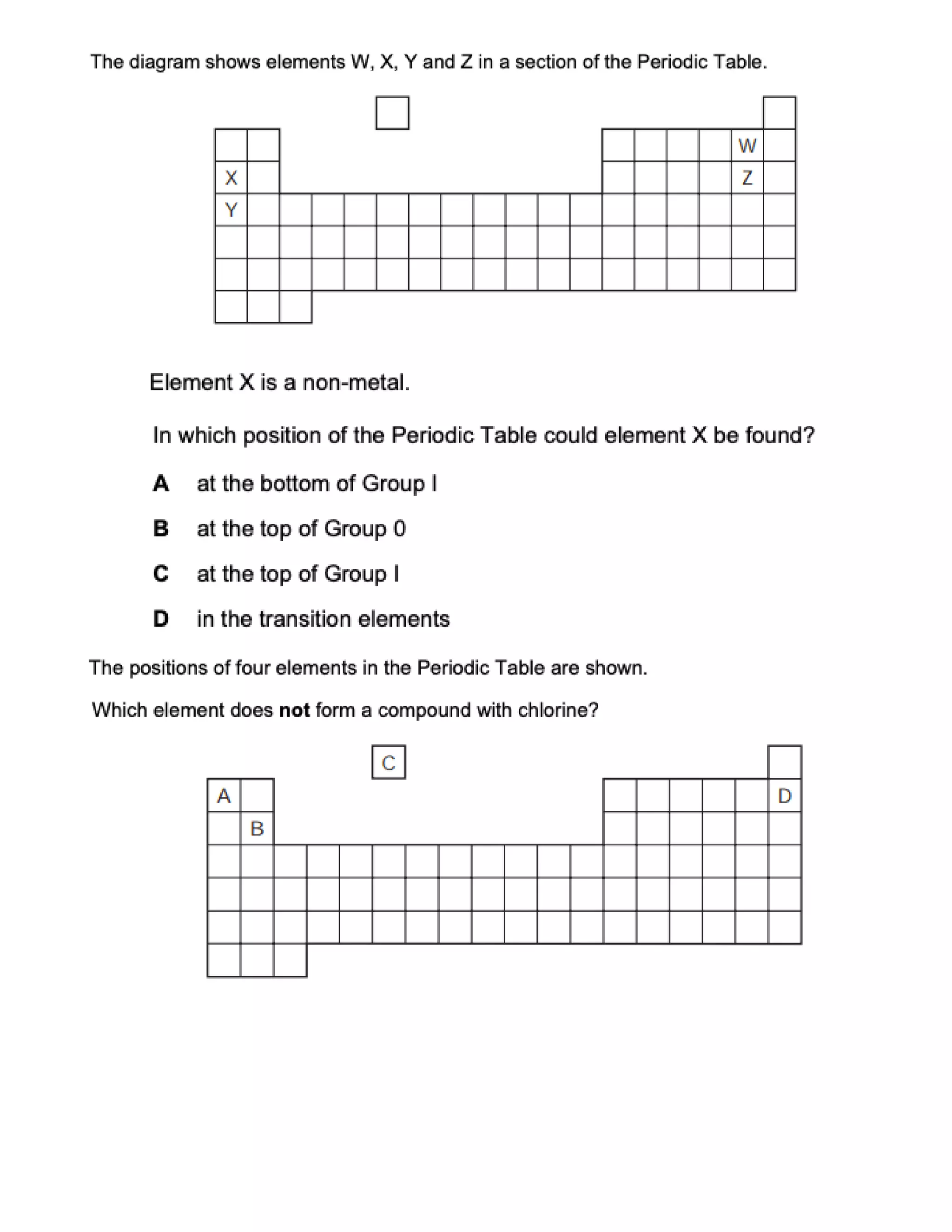
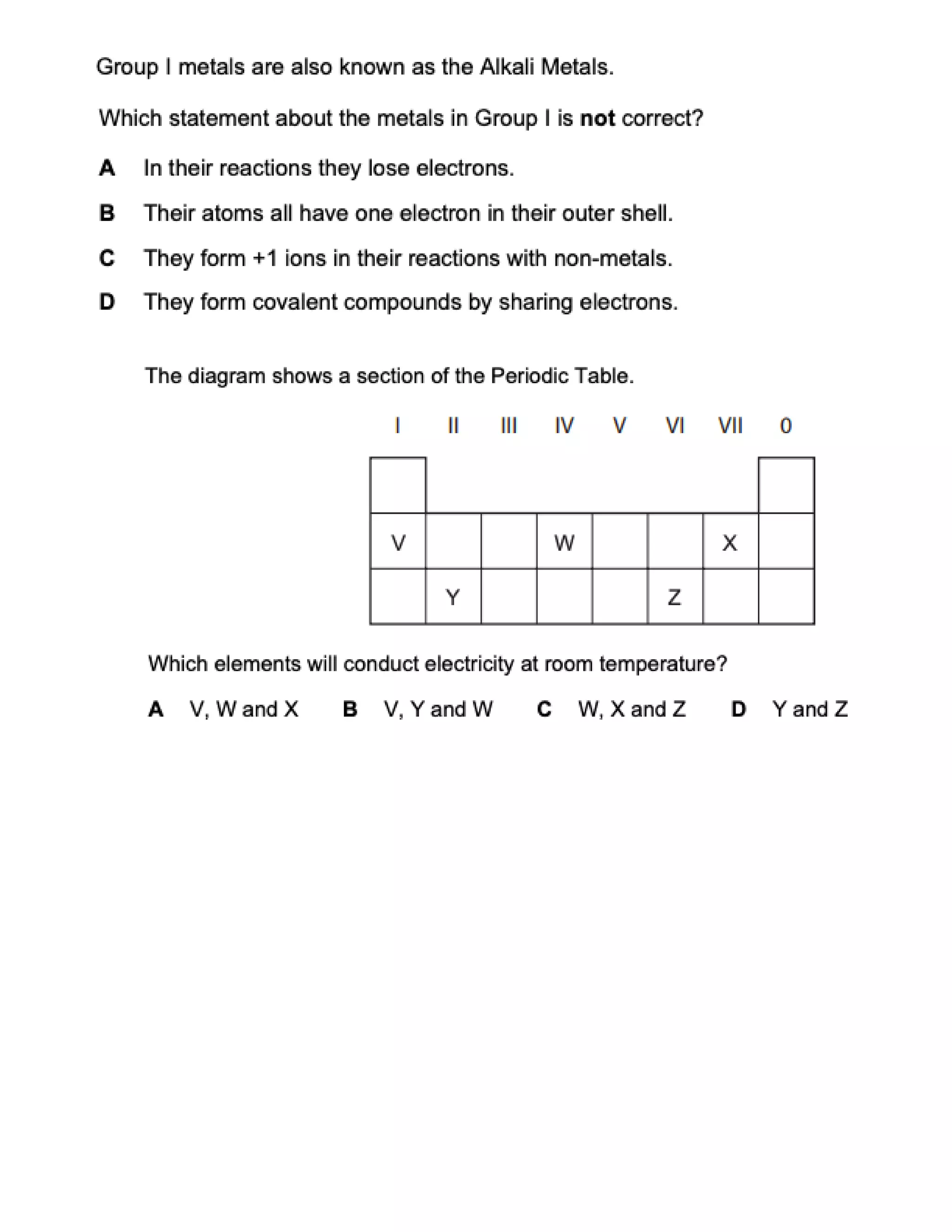
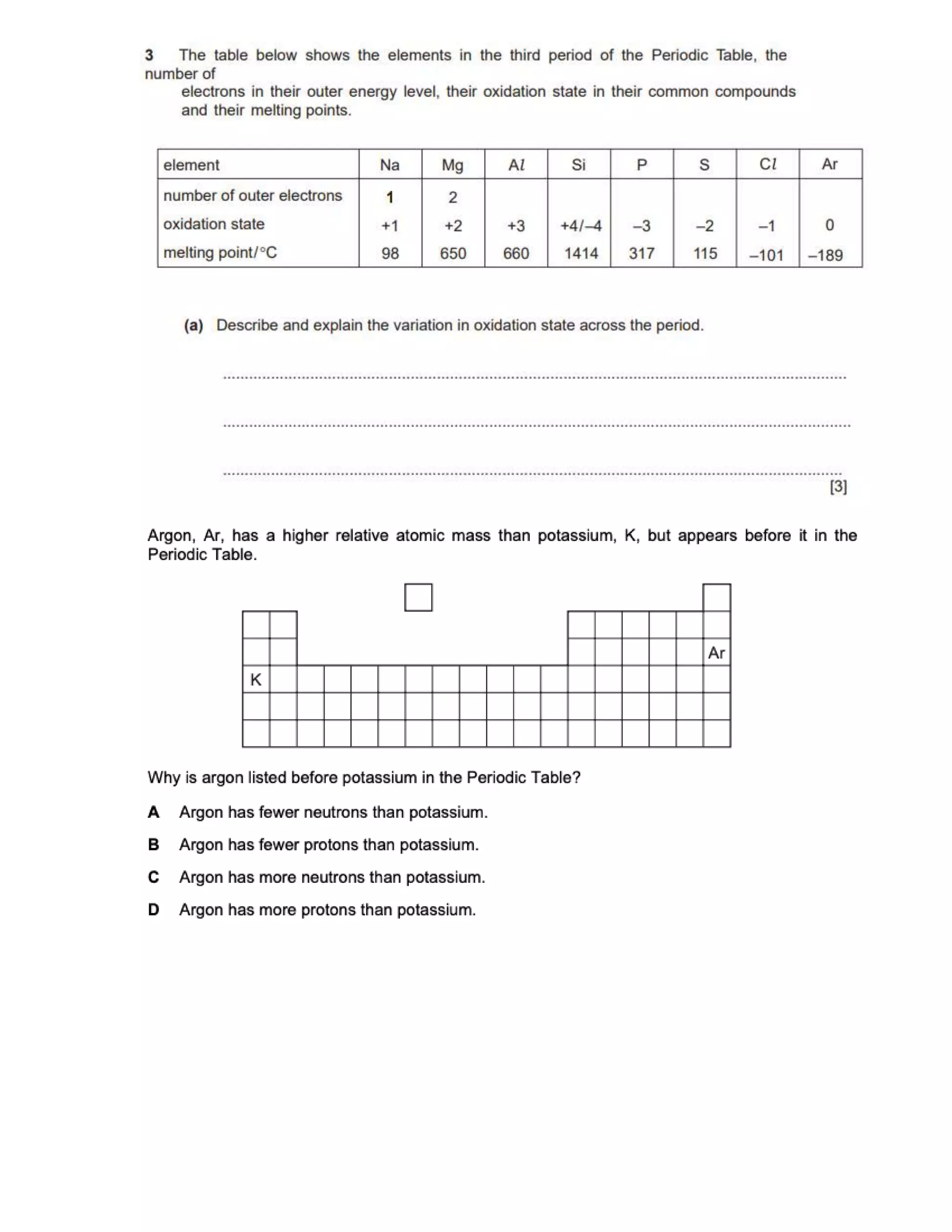
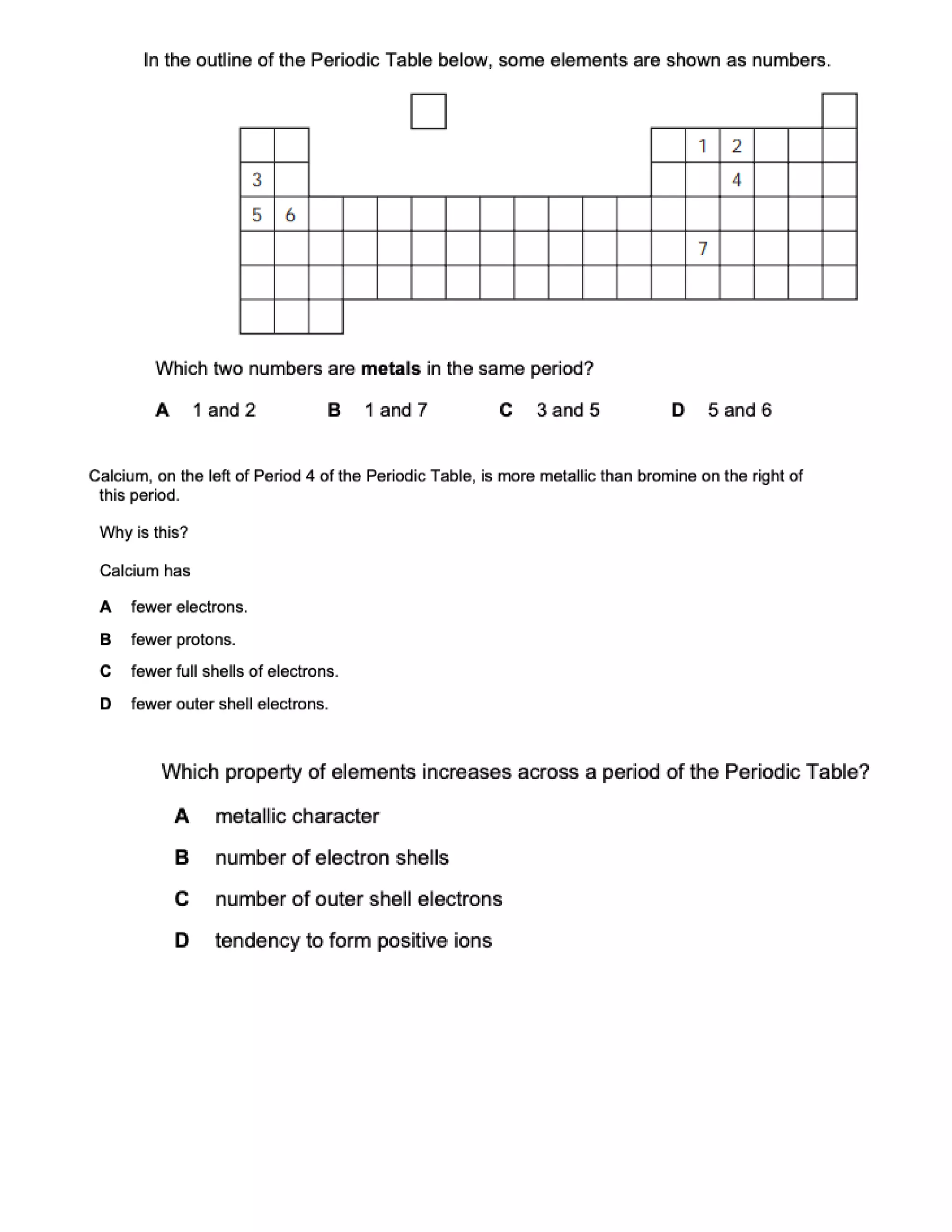
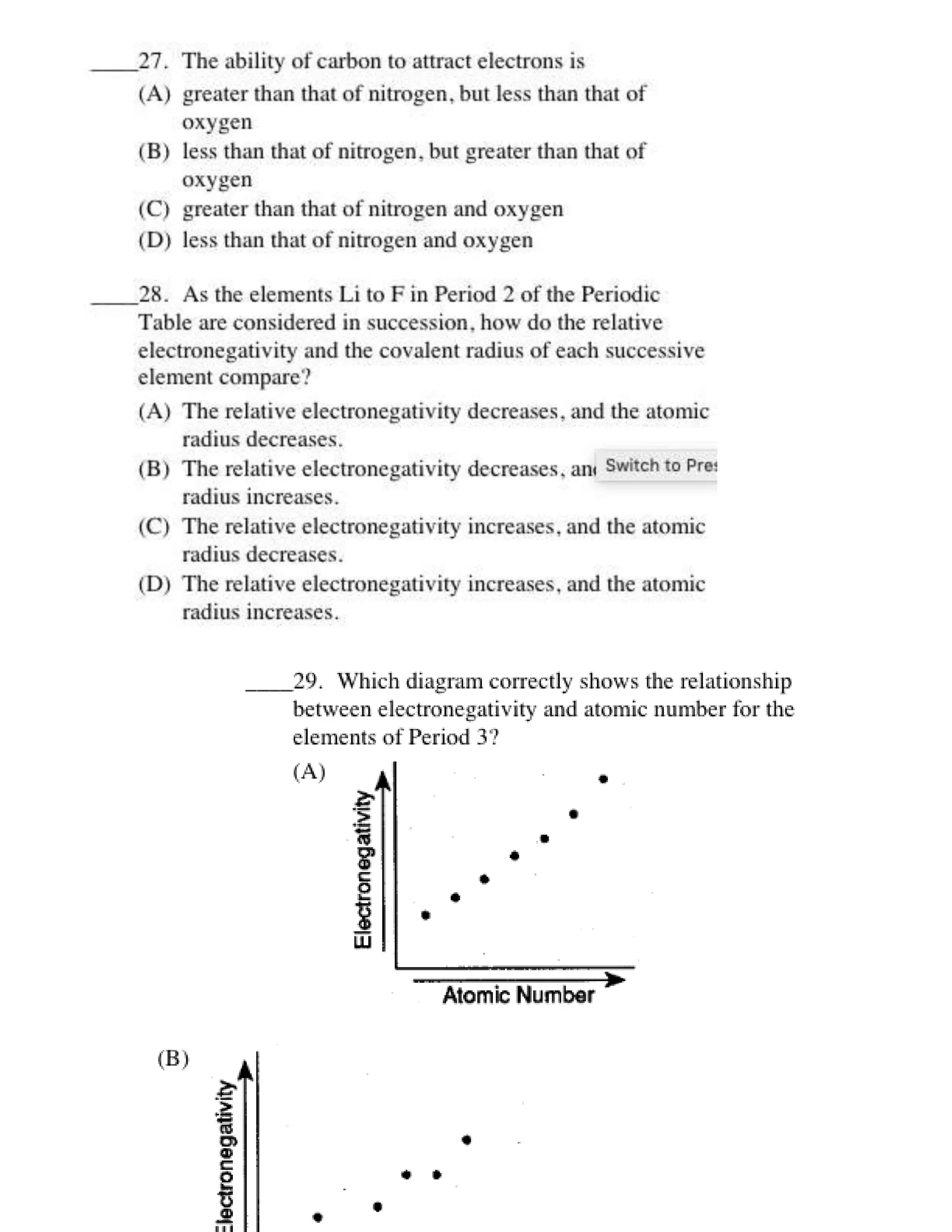
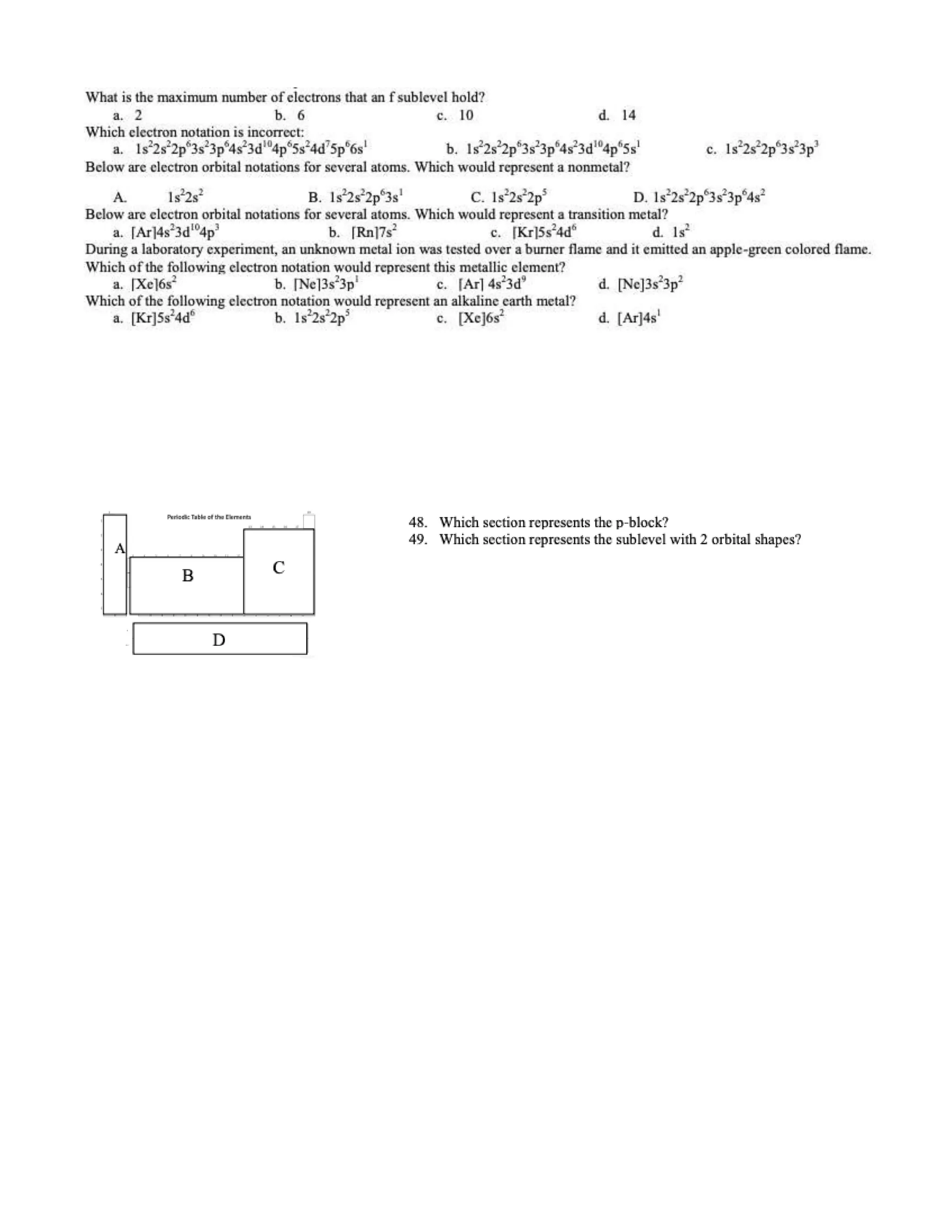
This document contains multiple choice questions about atomic structure and chemical bonding. It tests knowledge of subatomic particles, isotopes, ion formation, ionic and molecular compounds, polyatomic ions, and acids. The questions cover naming ions and compounds correctly, writing chemical formulas, identifying cation and anion pairs that form ionic compounds, and distinguishing between ionic and molecular compounds.