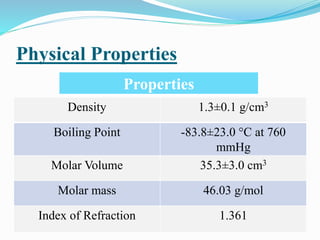
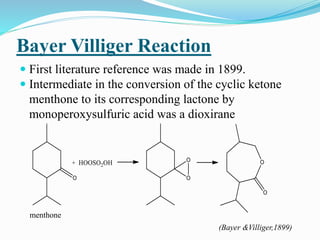
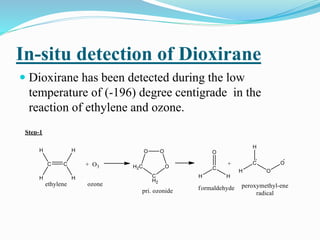
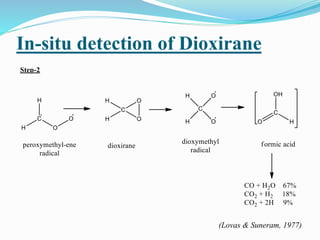
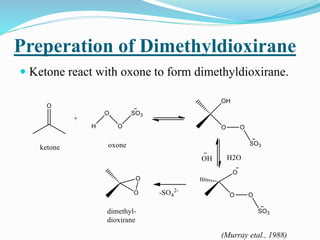
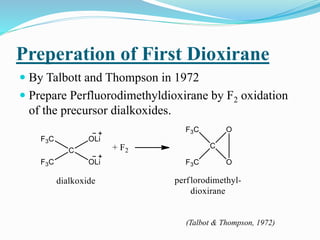
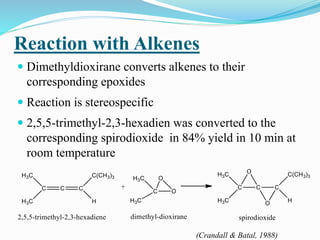
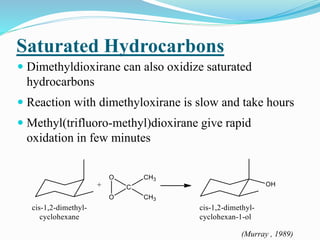
This document summarizes key information about dioxirane, the smallest cyclic organic peroxide. Dioxirane has an unstable structure with a long O-O bond length of 1.516Å. It was first detected in situ in 1977 and prepared in the laboratory in 1972. Dioxirane's most common reaction is oxygen atom transfer, such as converting alkenes to epoxides. It has applications as an oxidizing agent in organic synthesis and disinfection.