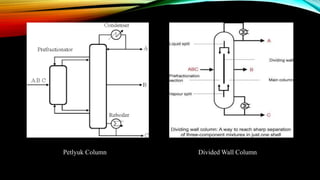
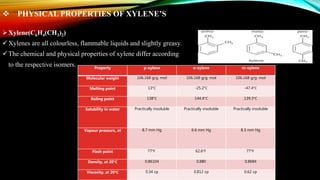
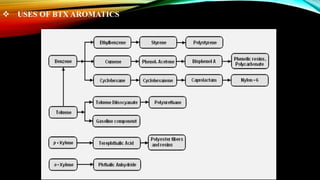
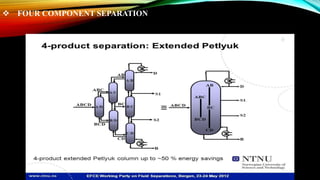
This document provides an overview of a student project to simulate the design of a divided wall column for separating reformate. Reformate is a mixture of hydrocarbons including benzene, toluene, xylene, and ethylbenzene. A divided wall column can save 20-40% of energy compared to conventional distillation columns. The student will perform material and energy balances, equipment design, and cost estimation to simulate the column. A literature review discusses the history and applications of divided wall columns in chemical plants.