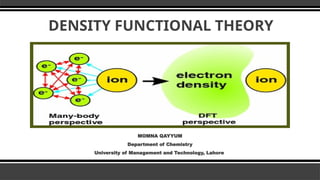
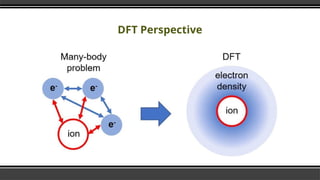
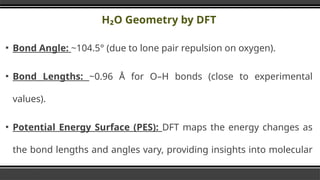
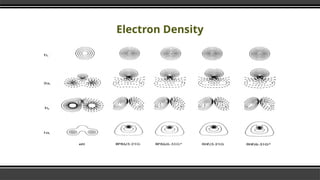
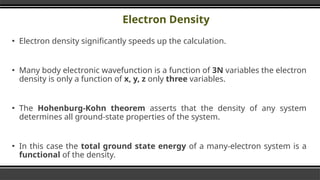
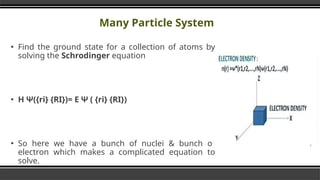
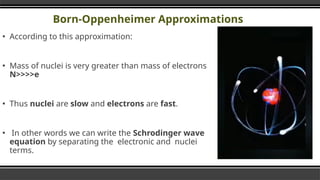
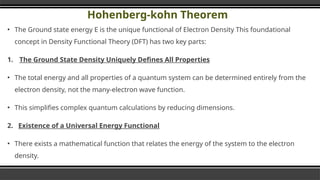
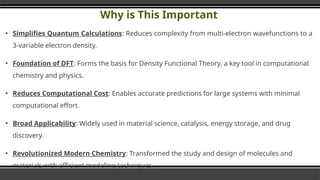
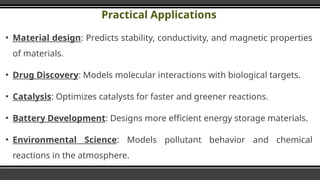
The document provides an overview of Density Functional Theory (DFT), a quantum-mechanical simulation method for calculating properties of atomic systems. It details the principles behind DFT, including the importance of electron density and the Hohenberg-Kohn theorem, which states that the ground state properties of a system can be determined from its electron density. The document also discusses practical applications of DFT in fields such as molecular dynamics, spectroscopy, and material science.







![Hohenberg-kohn Theorem 1
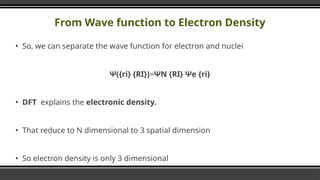
▪ The Ground state energy E is the unique functional of
Electron Density
E = E 。 [po(r)]
▪ where p (r) represents the density function which itself is a
function of position (r).](https://image.slidesharecdn.com/densityfunctionaltheoryoverview-241215172219-46ba724c/85/Density-Functional-Theory-DFT-Overview-pptx-21-320.jpg)
![▪ The electron density that minimizes the energy of the
overall functional is the true ground state electron density:
E[p(r)]>E.[p.(r)]
Hohenberg-kohn Theorem 2](https://image.slidesharecdn.com/densityfunctionaltheoryoverview-241215172219-46ba724c/85/Density-Functional-Theory-DFT-Overview-pptx-22-320.jpg)