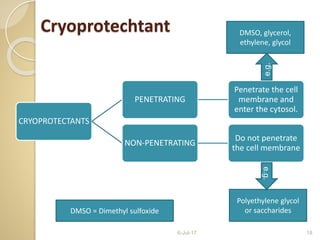
Cryopreservation is a technique for preserving biological material at very low temperatures, discovered by Ernest Polge in 1949. Its applications include long-term conservation of disease-free plants, endangered species, and preservation of microbiological cultures. Recent advancements favor vitrification over slow cooling due to its cost-effectiveness and handling ease.