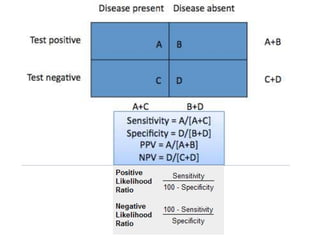
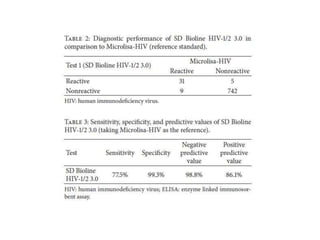
This study evaluated the performance of rapid diagnostic tests (RDTs) compared to ELISA for HIV screening. A total of 787 patient sera were tested using three different RDTs and ELISA, with western blot as the reference standard. The first RDT missed identifying 9 HIV-positive samples that ELISA correctly identified, giving the RDT a sensitivity of 77.5% compared to ELISA. The RDT also had 5 false positive results, giving it a specificity of 99.3% compared to ELISA. While the study provides the sensitivity and specificity of the RDT compared to ELISA, it does not provide confidence intervals for the results. The population studied represents asymptomatic individuals seeking HIV screening, so the results could likely be applied to screening