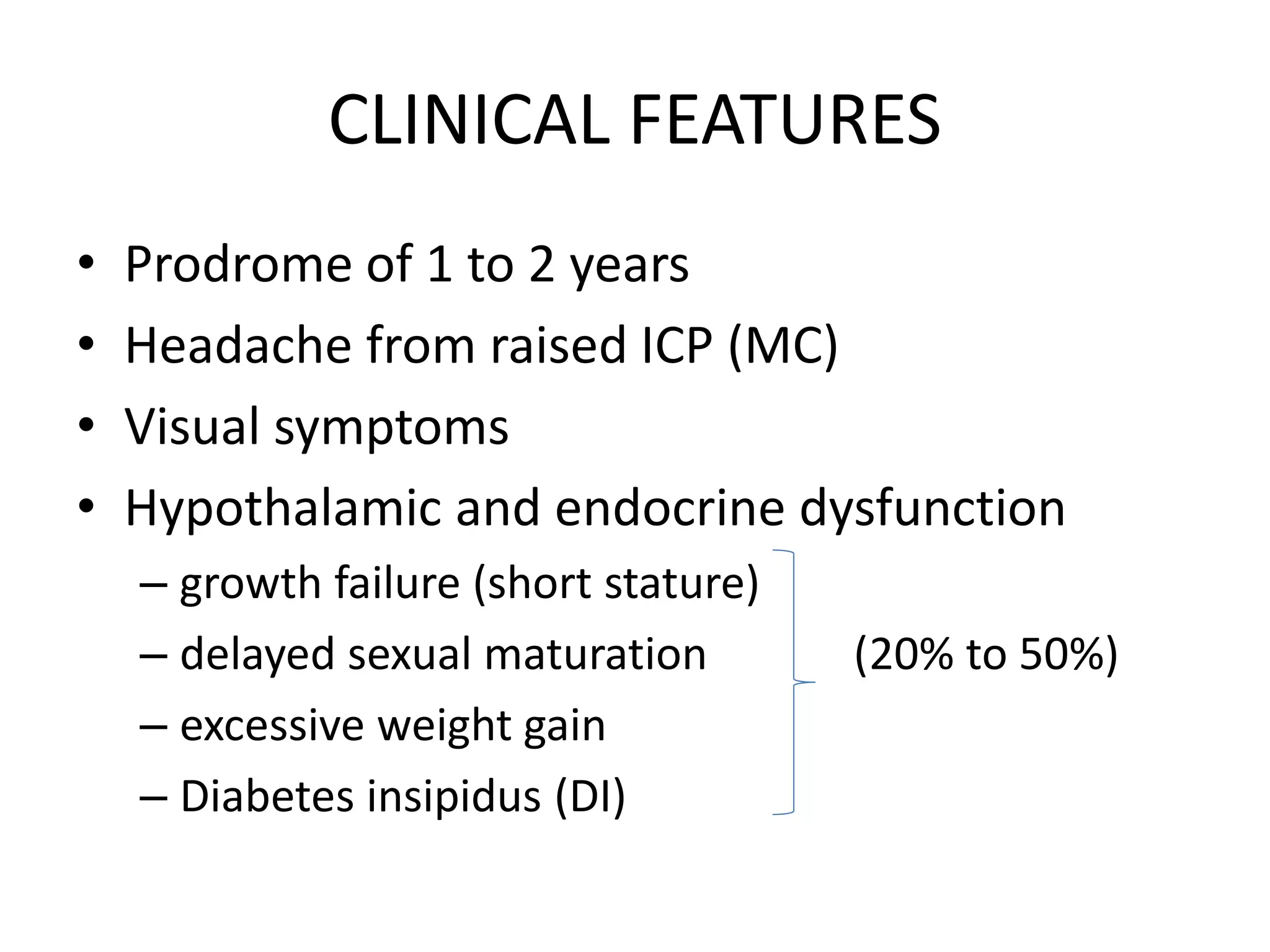
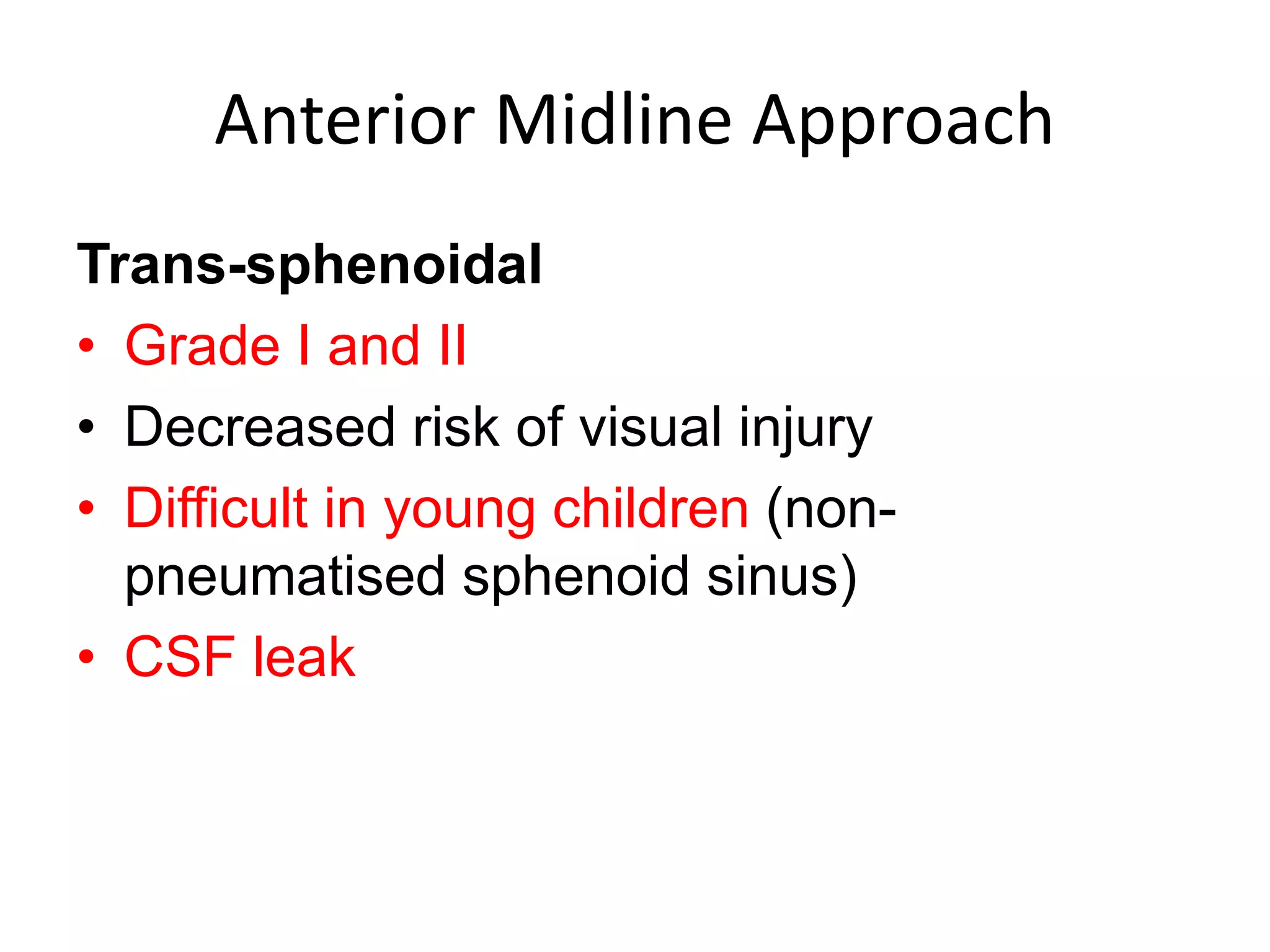
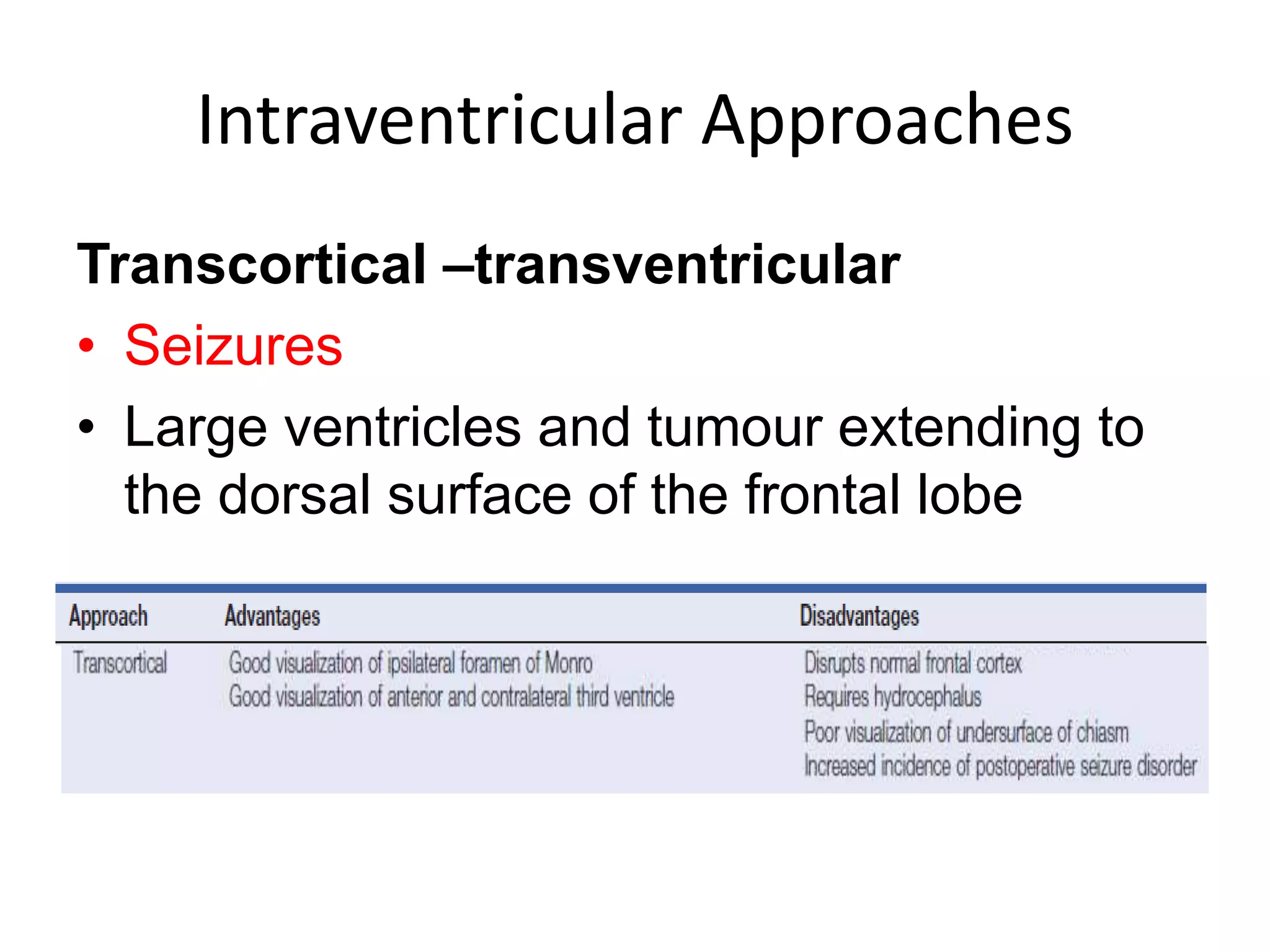
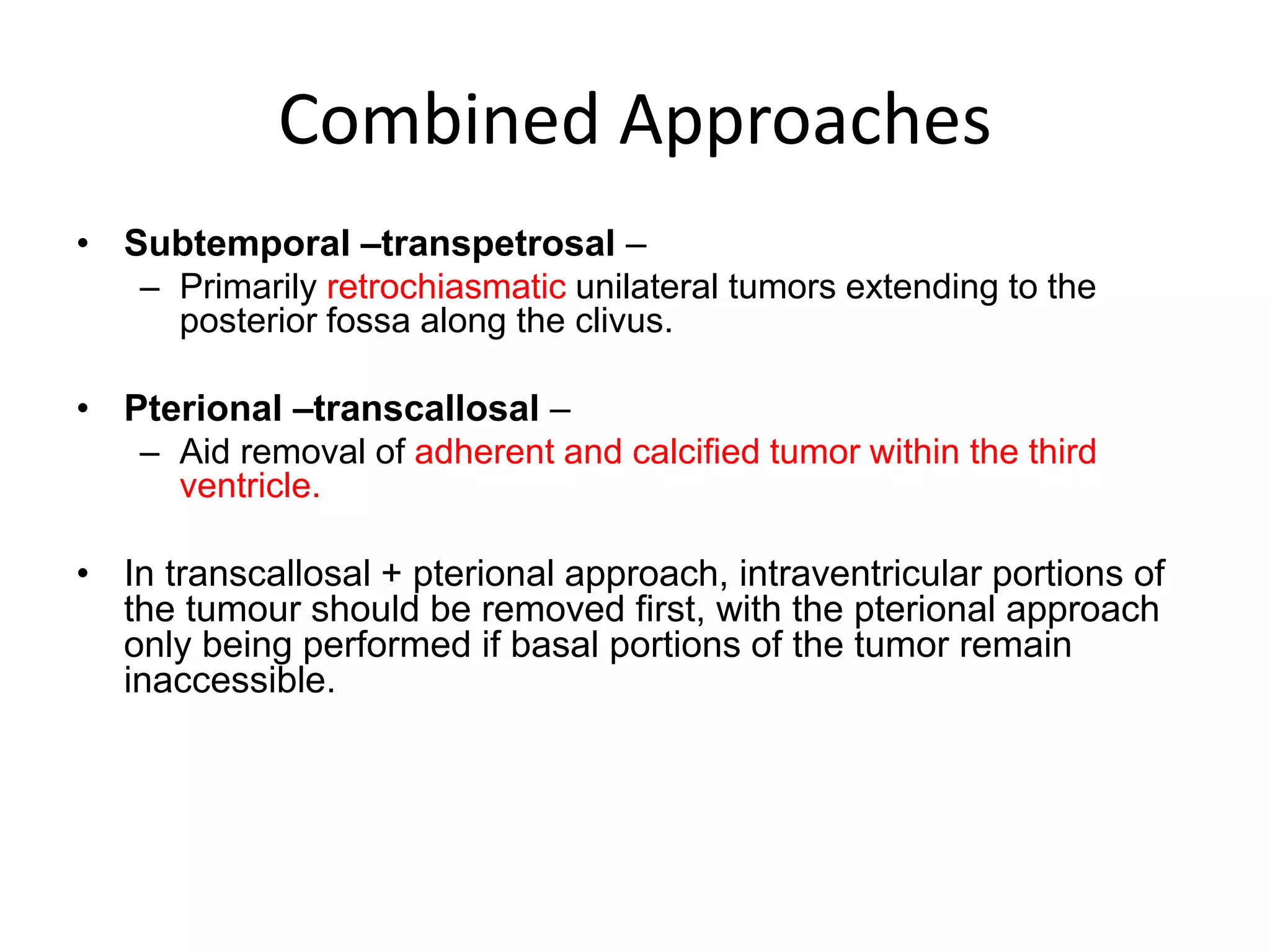
This document discusses craniopharyngioma, a rare brain tumor that arises near the pituitary gland. It has a bimodal age distribution in children and adolescents. Surgical removal is the primary treatment but can be challenging due to the tumor's location near vital structures. The goals of surgery are complete removal while preserving neurological function. Radiation and chemotherapy may also be used for residual or recurrent tumors. Ongoing monitoring is important due to the risk of the tumor returning after initial treatment.