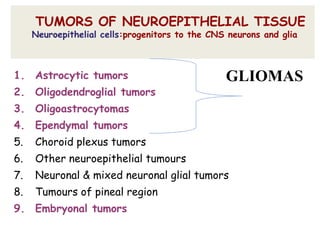
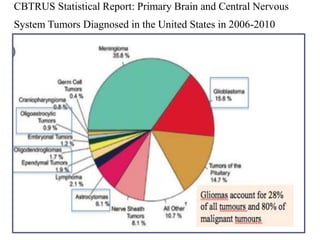
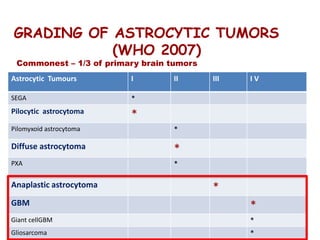
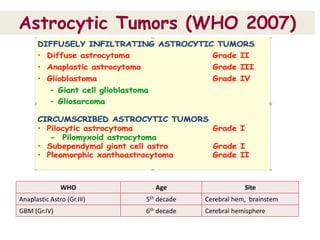
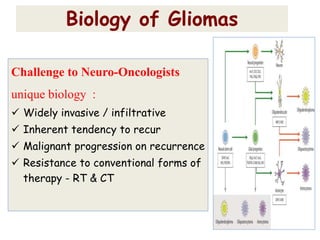
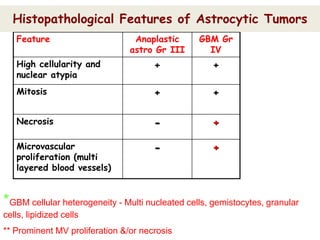
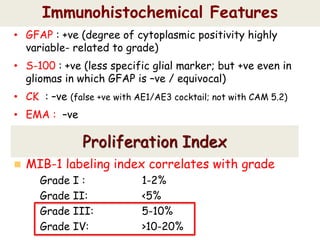
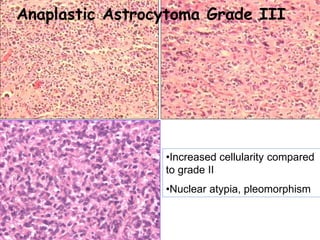
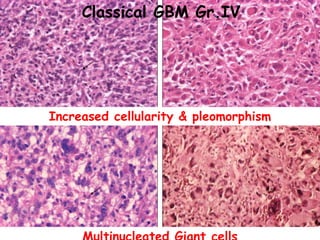
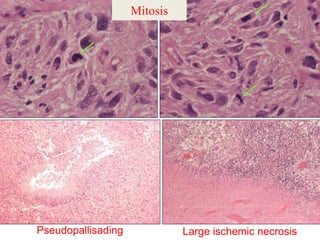
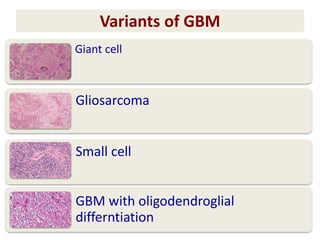
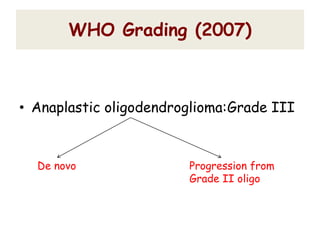
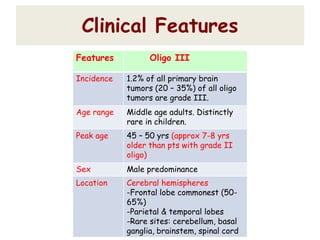
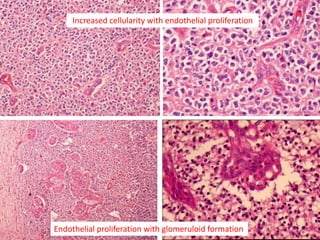
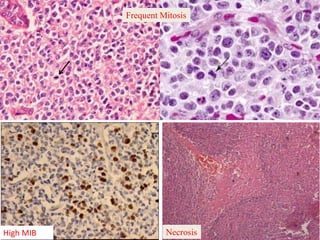
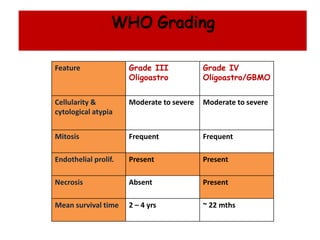
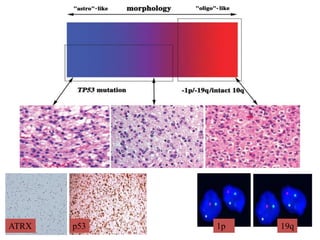
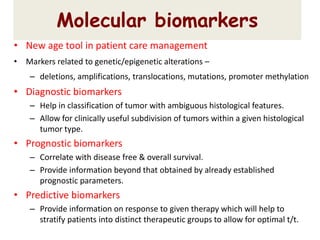
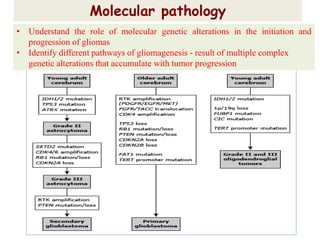
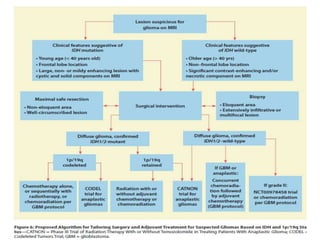
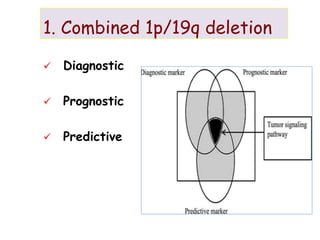
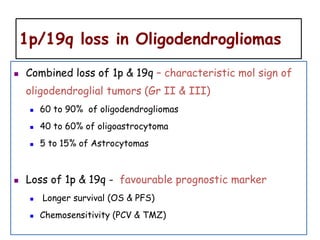
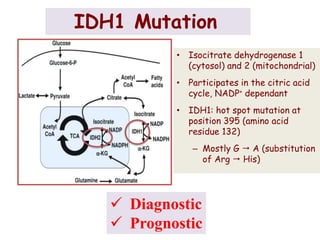
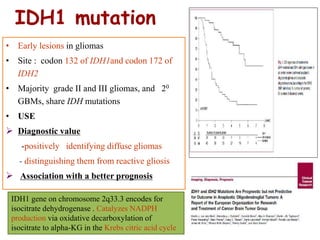
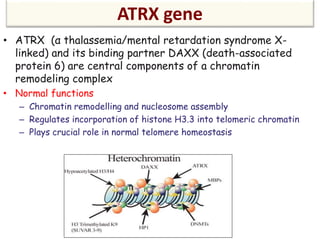
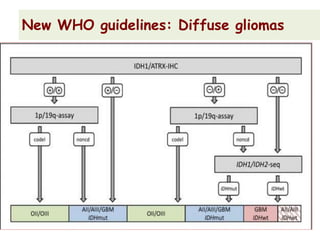
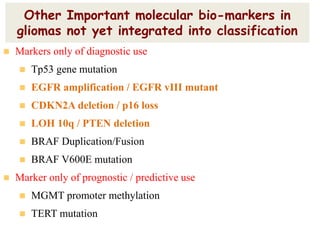
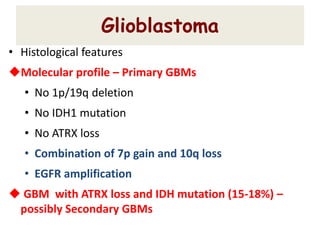
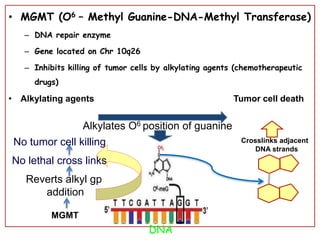
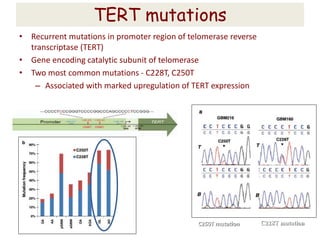
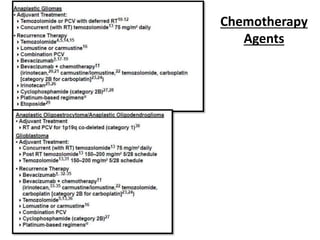
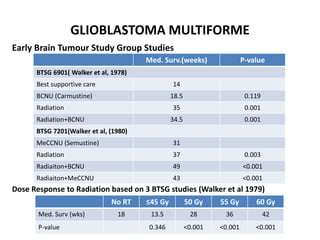
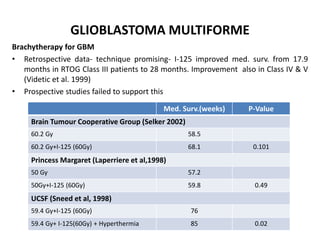
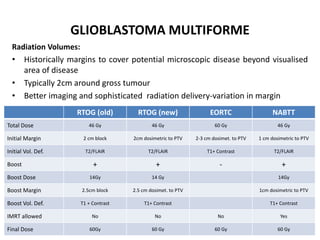
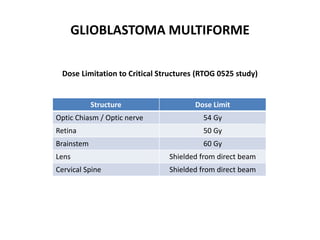
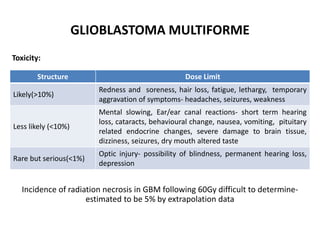
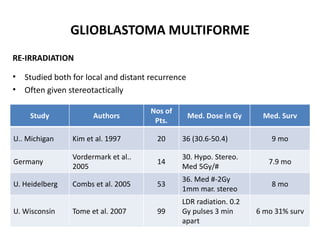
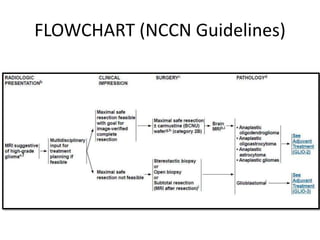
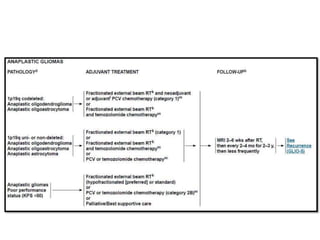
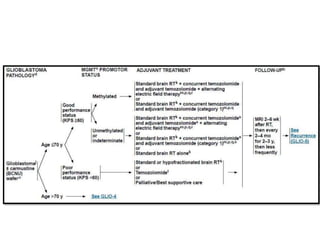
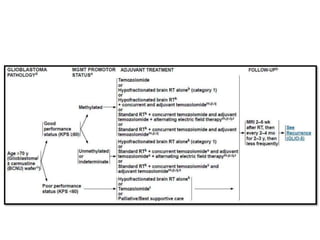
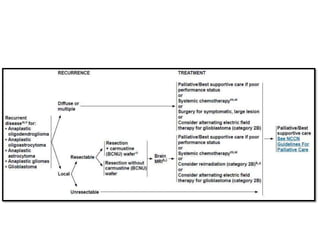
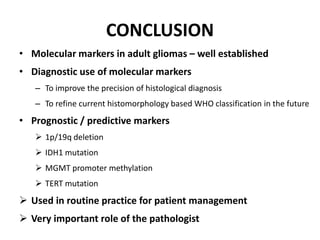
This document discusses the classification and molecular markers of brain tumors according to the WHO. It focuses on gliomas, specifically glioblastoma multiforme and anaplastic astrocytoma. It describes the histopathological and molecular features used to classify these tumors, including markers like IDH1 mutation, 1p/19q codeletion, and ATRX mutation. Molecular testing is becoming increasingly important for diagnosis, prognosis, and predicting response to therapies of diffuse gliomas. The document also discusses treatment approaches including surgical resection and chemotherapy.