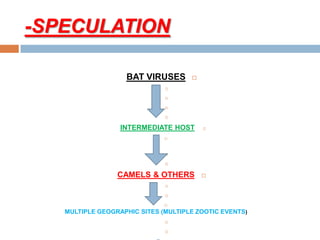
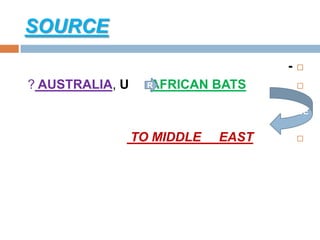
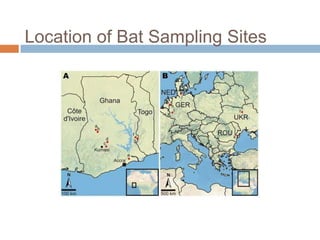
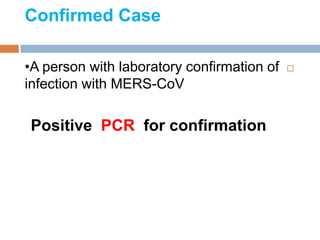
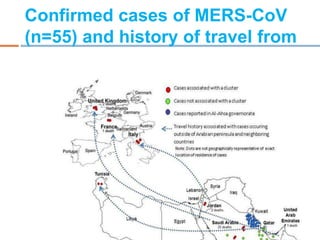
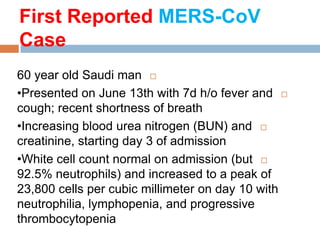
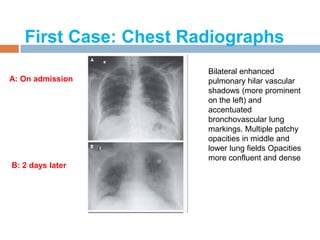
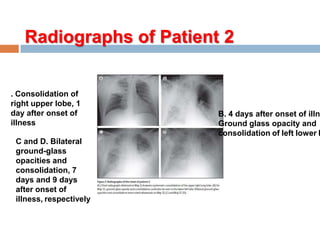
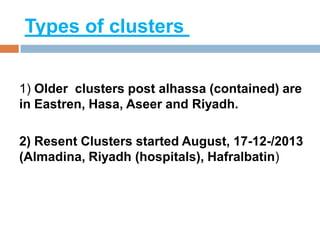
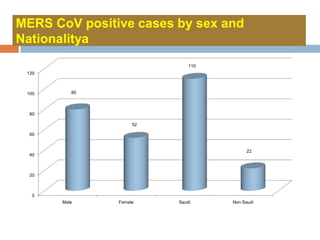
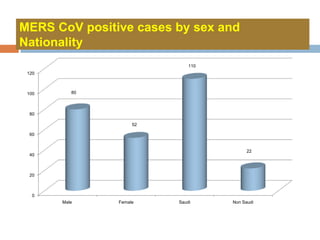
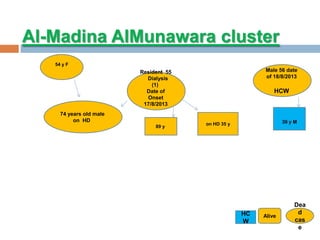
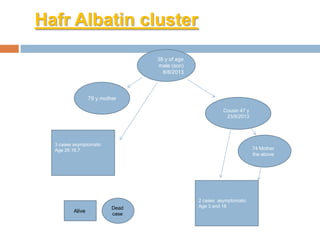
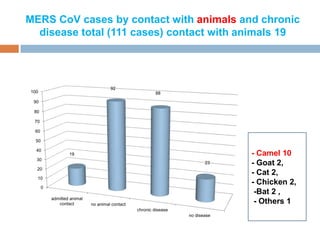
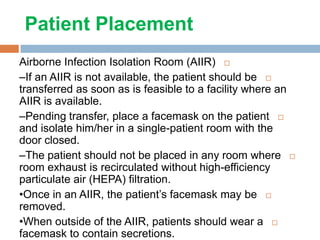
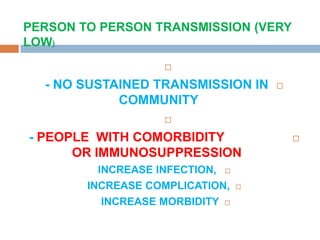
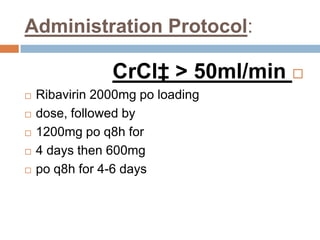
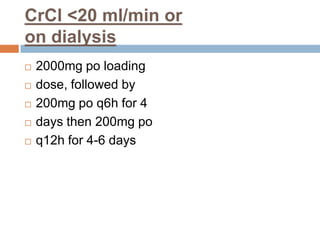
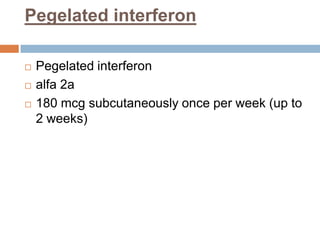
The document provides an overview of Middle East Respiratory Syndrome Coronavirus (MERS-CoV), first identified in 2012, including its origins, transmission, and clinical implications. It reports on confirmed cases and deaths across various countries, the virus's behavior within the human body, and infection control measures for healthcare professionals. Current treatment options are limited, with a lack of vaccines, and recommendations for those traveling to affected regions are outlined to mitigate risk.