The document covers various aspects of coordination compounds in inorganic chemistry, including Werner’s coordination theory, the concept of ligands, and the classification based on denticity. It discusses the formation, nomenclature, and stability of coordination complexes, as well as chelating ligands and the significance of complex geometry. Additionally, it reviews experimental verifications of Werner’s theory and its limitations.


![CO-ORDINATION COMPOUNDS: Co-ordination compounds are
molecular compounds which do not dissociate in to all the constituent ions in
aqueous solution. They consist-
(i) Simple cation + complex anion
Example : K3[Fe(CN)6] 3K+
+ [Fe(CN)6]3-
simple cation complex anion
(ii) Complex cation + simple anion
[Cu(NH3)4]SO4 [Cu(NH3)4]2+
+ SO4
2-
complex cation simple anion
(iii) Complex cation + complex anion
[Pt(NH3)4][Cu(CN)4] [Pt(NH3)4]2+
+ [Cu(CN)4]2-
complex cation complex anion
(iv) Complex neutral molecules
Ni(CO)4, Fe(CO)5](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-4-320.jpg)
![COMPLEX ION: A complex ion consists -
(i) Co-ordination sphere shown by [ ].
(ii) Central metal ion (M)
(iii) Oxidation state (OS) of central metal ion
(iv) Ligands (L)
(v) Co-ordination number (CN) of metal ion
Example: [Cu(NH3)4]2+
Metal ion: Cu2+
; OS of Cu: +2
Ligands: NH3; CN of Cu(II) =4
In complex ion-
(i) The central metal ion is Lewis acid and can accept pair of electrons.
(ii) Ligands are Lewis base and can donate pair of electrons.
(iii) Co-ordinate bonds are formed between ligands and metal ion](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-5-320.jpg)

![CHELATING LIGANDS: Bidentate and poly dentate ligands form ring
compounds with the central metal ion. Such ligands are called chelating ligands
and the complex is called chelate.
Example: en (ethylenediammine), EDTA (ethylenediamminetetraacetic acid)
CHELATES: The ring or cyclic structure formed when a bidentate or
polydentate ligand is attached by two or more donor atom to the same central
metal ion, is called a chelate.
Such ligands which bind the metal atom through two or more donor atoms are
called chelating ligands.
Example : [Cu(en)2]2+
[en (ethylene diammine) is a bidentate ligand]
CH2 H2N NH2 CH2 CH2 H2N NH2 CH2
+ Cu2+
+ Cu
CH2 H2N NH2 CH2 CH2 H2N NH2 CH2
Bis(ethylenediammine)copper(II) ion
2+ 2+](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-8-320.jpg)
![IMPORTANCE OF CHELATES :
Chelates are more stable than non-chelates.
The stability of complexes also depend upon the following factors-
1. Number of chelate rings: A chelate in which more number of rings are
formed is more stable i.e. larger the number of chelate rings in the
complexes, greater is its stability.
Example: stability is in the order:
[Ni(en)3]2+
> [Ni(en)2(H2O)2]2+
> [Ni(en)(H2O)4]2+
> [Ni(H2O)6]2+
3 rings 2 rings 1 ring no ring
2. Size of the chelate rings: Metal chelates with five membered rings have been
found to be more stable.
Example: [Cu(en)2]2+
has two five membered rings.](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-9-320.jpg)
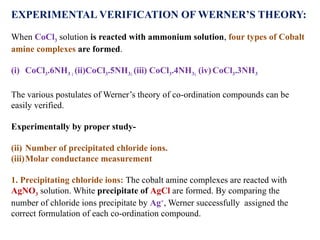
![CoCl3.6NH3 3 mols of AgCl ppt
CoCl3.5NH3 2 mols of AgCl ppt
CoCl3.4NH3 1 mol of AgCl ppt
CoCl3.3NH3 No ppt
Therefore, the number of ionizable chlorides ions are 3Cl-
, 2Cl-
, Cl-
and zero,
respectively. Hence, the formula of complexes will be-
(i) [Co(NH3)6]Cl3 (ii) [Co(NH3)5Cl]Cl2
(iii) [Co(NH3)4Cl2]Cl (iv) [Co(NH3)3Cl3]](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-13-320.jpg)
![Cobalt amines Molar conductance
(Ω-1
cm2
mol-1
)
No. of ions/
charge
Structural
formula
CoCl3.6NH3 395 4 (+3, -3) [Co(NH3)6]Cl3
CoCl3.5NH3 265 3 (+2, -2) [Co(NH3)5Cl]Cl2
CoCl3.4NH3 108 2(+1, -1) [Co(NH3)4Cl2]Cl
CoCl3.3NH3 0 0 [Co(NH3)3Cl3]
In all the four amines PV of Co = +3 satisfied by 3Cl-
ions
SV of Co =6
Structure = octahedral
(ii) Molar conductance measurement: This method gives number of ions and
charge on ions. The experimental value and result are as follow-
LIMITATIONS OF WENER’S THEORY:
This theory doesn’t explain-
(i) Stability of complexes
(ii) Magnetic properties, colour and electronic spectra of complexes
(iii)Nature of ligands and the types of orbitals involved in the bonding.](https://image.slidesharecdn.com/1stlectureonco-ordinationcompounds-241215045657-acb88fa2/85/1st-lecture-on-Co-ordination-compounds-pptx-14-320.jpg)