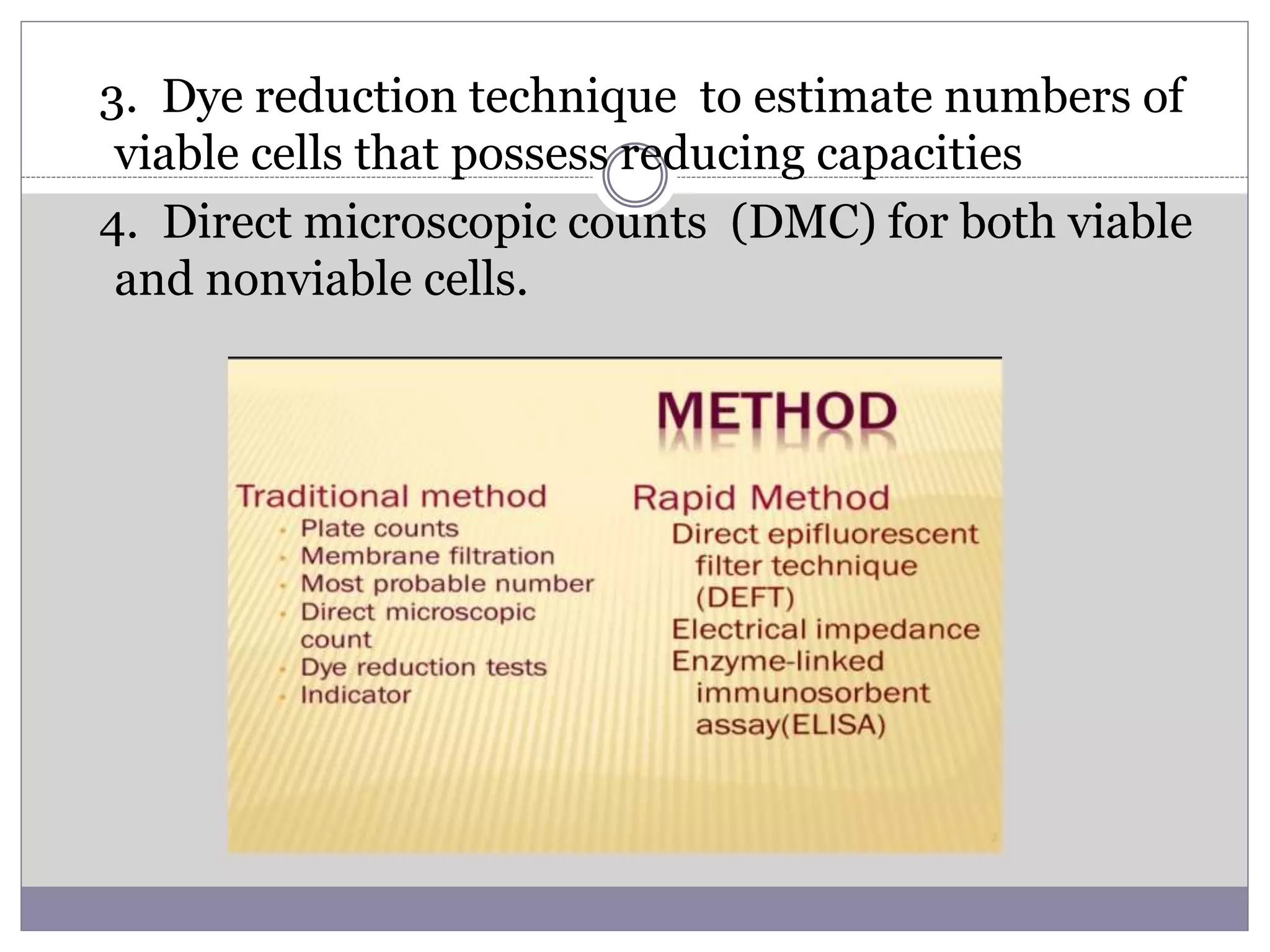
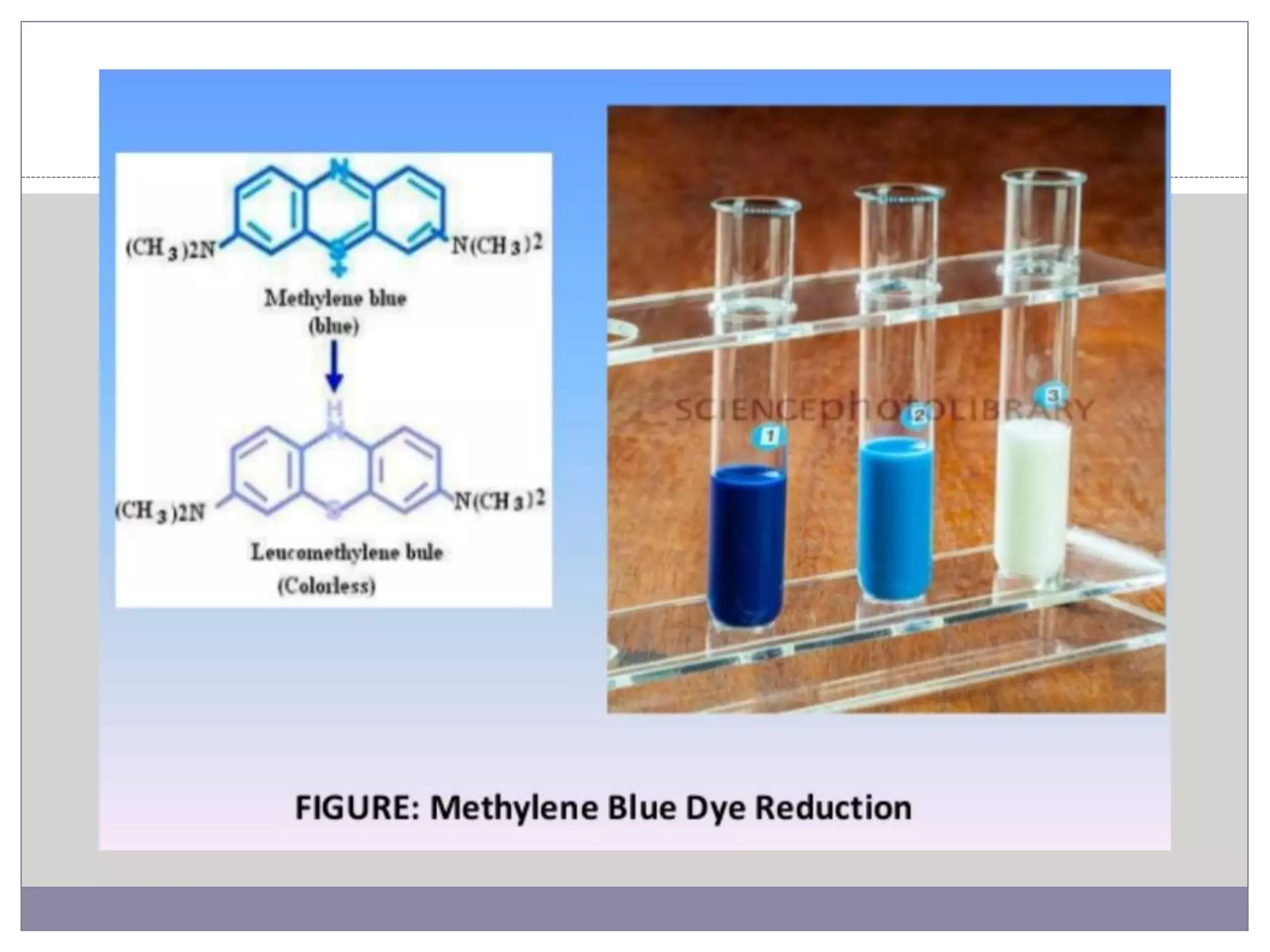
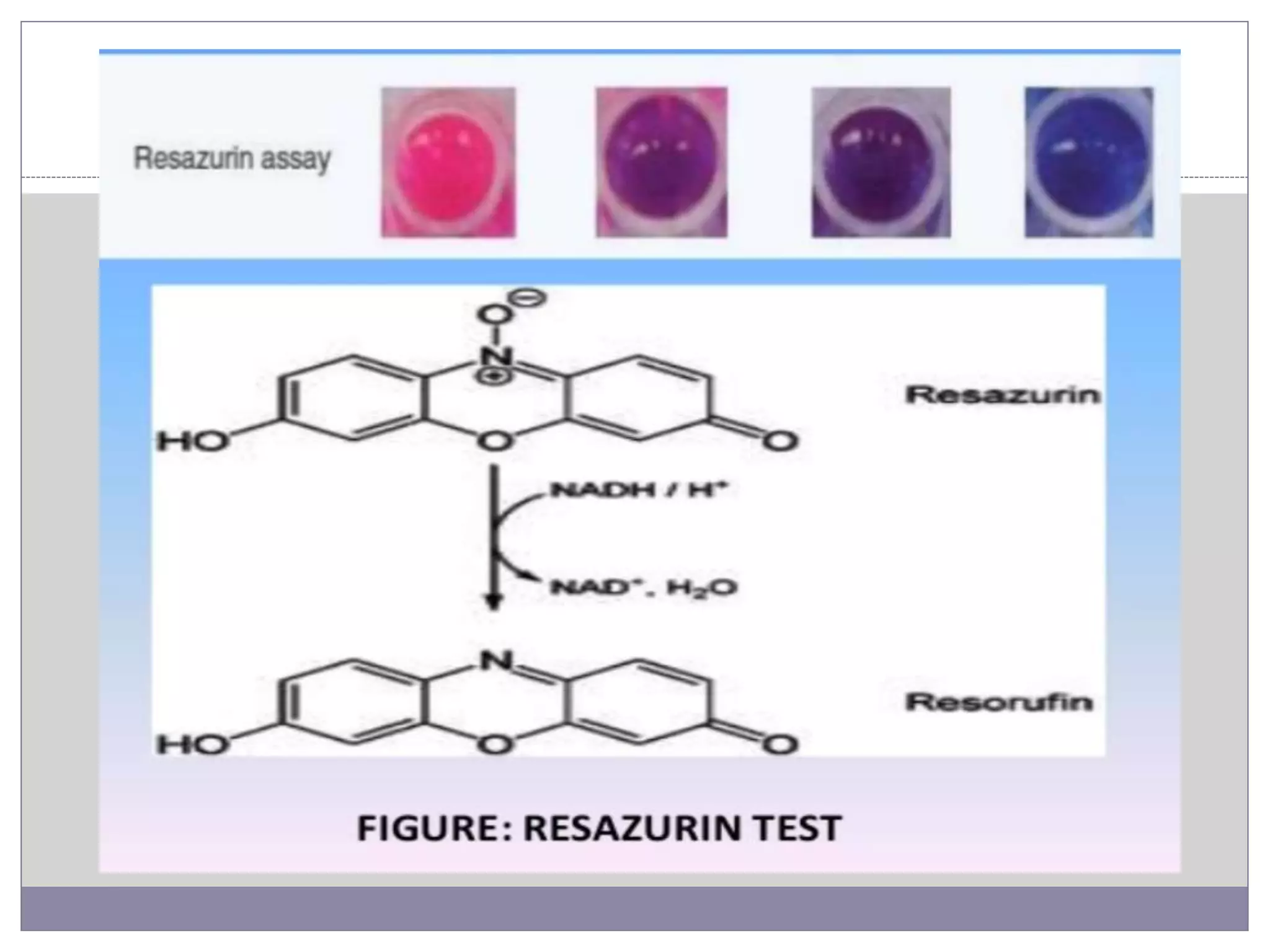
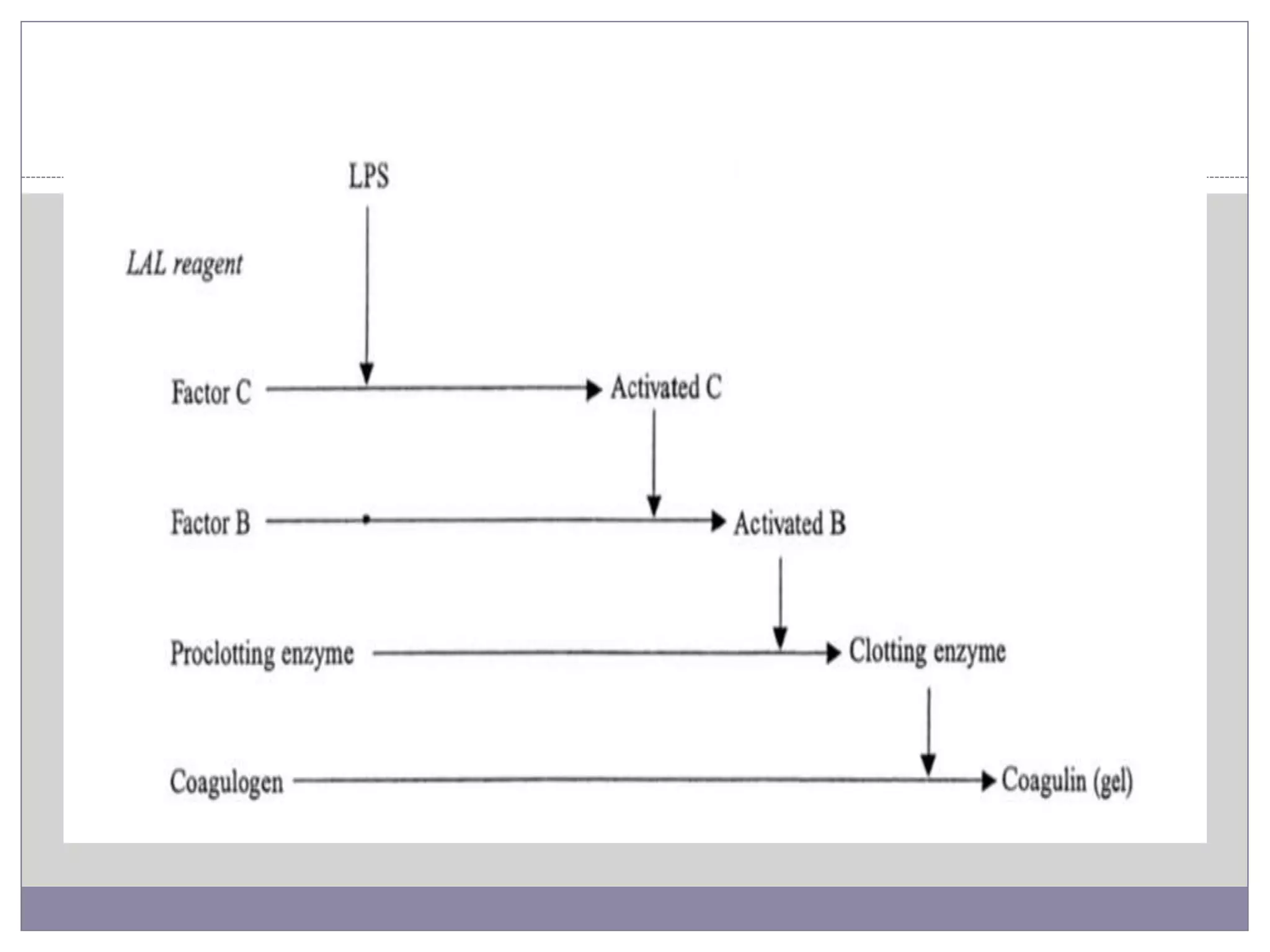
This document summarizes conventional and modern methods for the detection of spoilage and characterization of microorganisms in foods. It describes conventional methods such as standard plate counting, most probable number techniques, dye reduction, and direct microscopic counting. It then outlines several modern chemical methods like thermostable nuclease assays, Limulus amoebocyte lysate testing for endotoxins, and ATP bioluminescence. Biological methods like ELISA, PCR, and DNA probes are also covered. Finally, some physical detection methods involving biosensors, microcalorimetry, flow cytometry, and automated detection systems are presented.