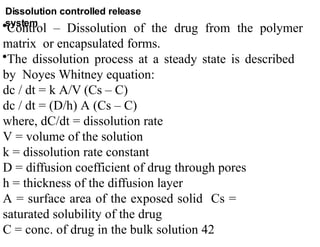
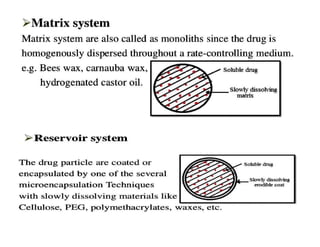
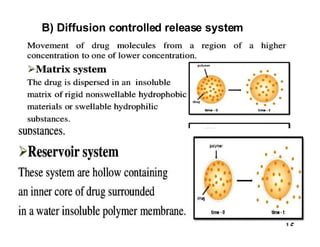
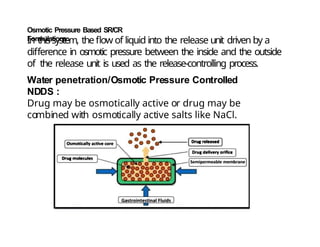
The document discusses drug delivery systems, highlighting the differences between conventional and novel drug delivery systems (NDDS). It explains the importance of controlled and sustained release mechanisms to maintain effective drug concentrations, detailing various approaches for designing such systems. Additionally, it covers topics like matrix and reservoir devices, diffusion-controlled systems, and factors influencing the design of controlled release formulations.