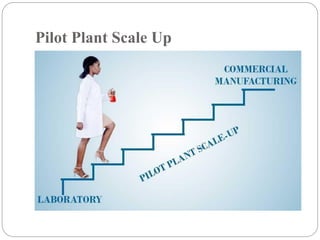
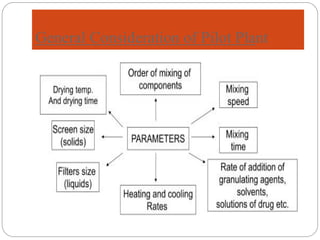
The document discusses the significance of pilot plant scale-up techniques in pharmaceutical manufacturing, emphasizing the need for intermediate batch scales to transform lab formulas into viable products. It outlines the objectives, applications, and general considerations for conducting pilot plant studies, including personnel, space, equipment, and quality assurance protocols. The pilot plant serves as an essential step to evaluate processes before full-scale production, ensuring the stability and efficacy of products.