Embed presentation







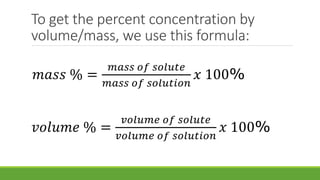
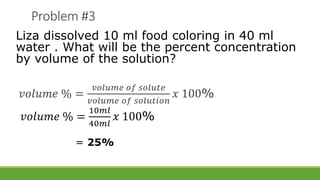
Concentration of solution refers to the relative amounts of solute and solvent. It can be expressed as percent by volume, which is the amount of solute in a given volume of solution in grams per 100 mL. It can also be expressed as percent by mass, which is the amount of solute in a given mass of solvent in grams per 100 grams of solution. Sample problems are provided to demonstrate how to calculate the concentration of a solution by determining the mass or volume of solute present using the total mass or volume of the solution and the given percentage concentration. Formulas are also provided to calculate mass percent and volume percent concentration.








