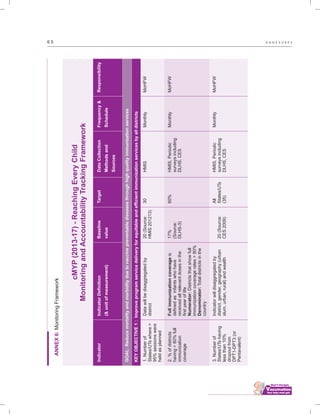
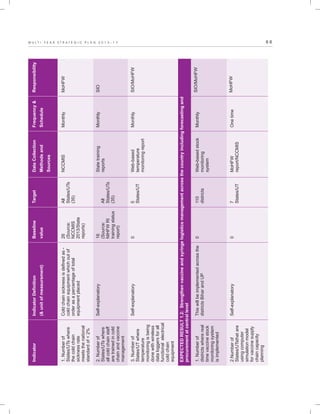
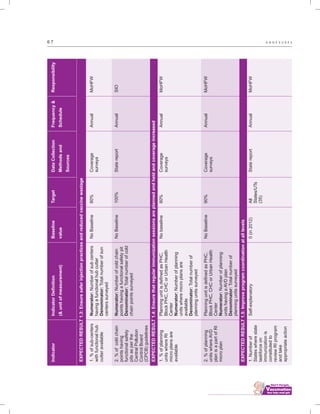
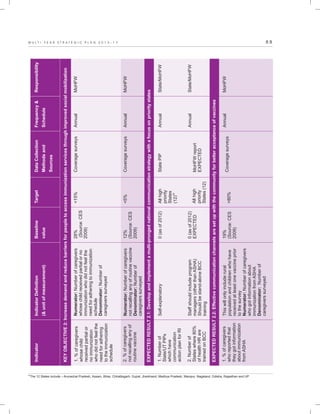
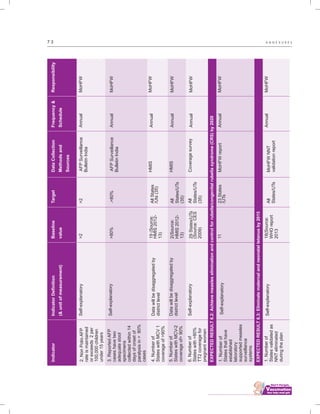
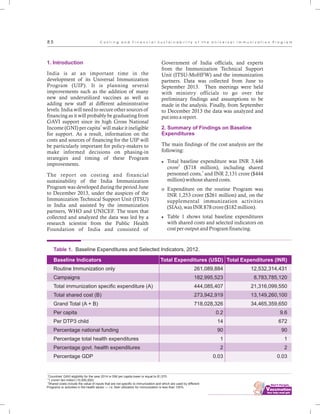
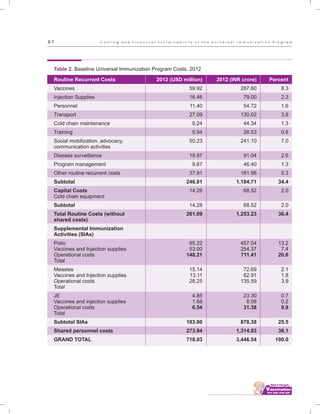
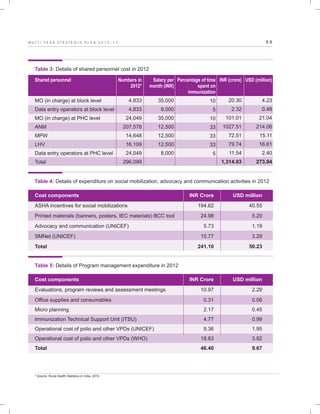
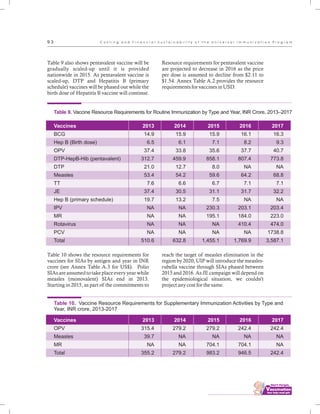
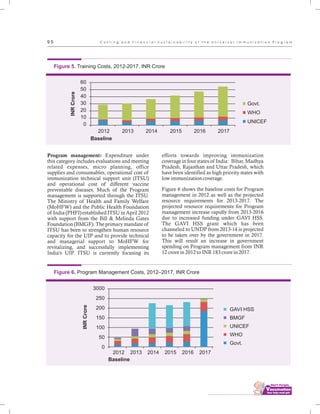
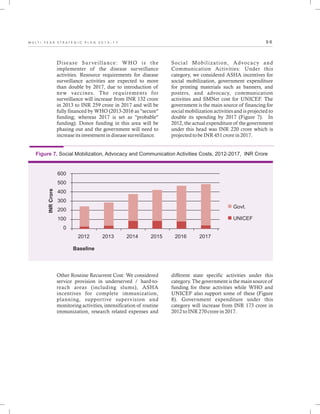
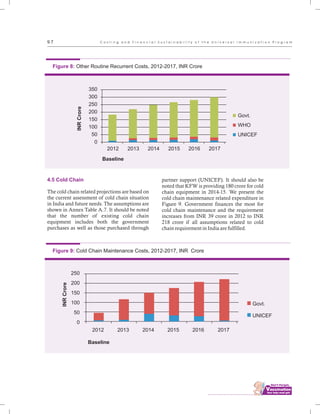
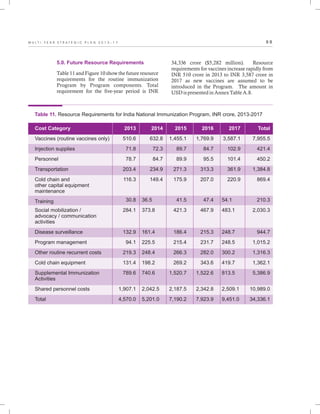
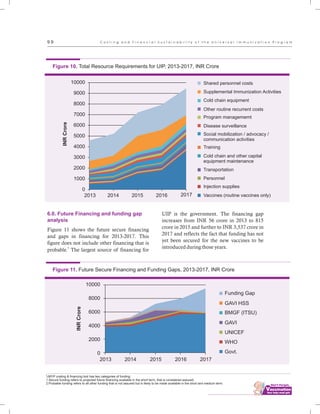
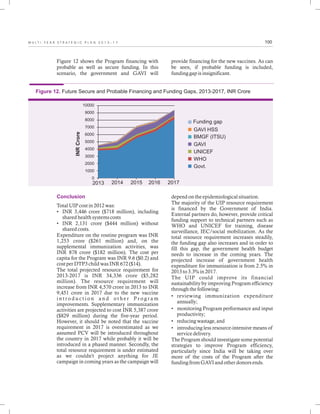
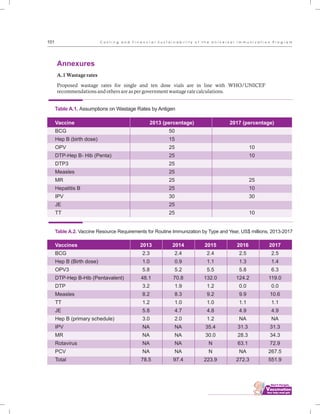
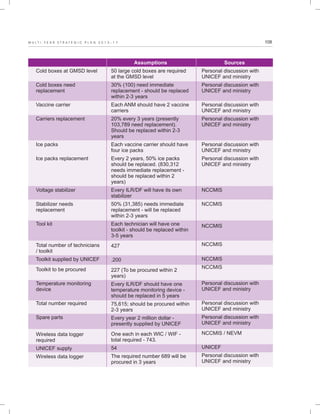
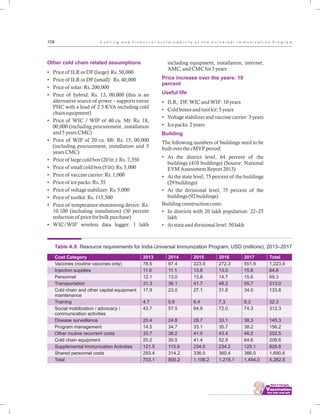
The document outlines the multi-year strategic plan for India's Universal Immunization Program (UIP) from 2013 to 2017, aimed at improving vaccine coverage and addressing vaccine-preventable diseases among children. It includes the program's background, current status, guiding principles, and monitoring and evaluation plans, along with a focus on financial sustainability. The plan is developed under the Ministry of Health and Family Welfare with contributions from various health sector stakeholders.