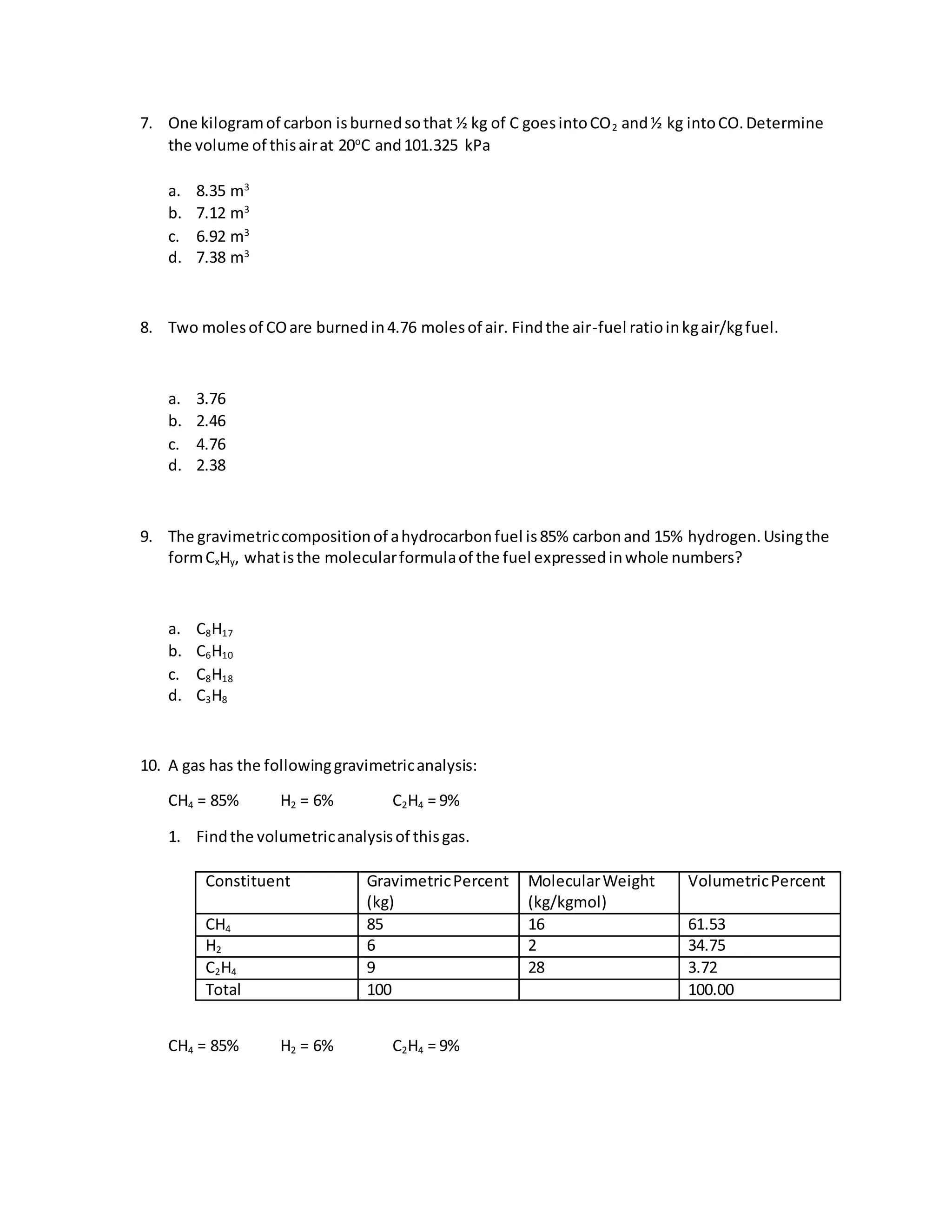
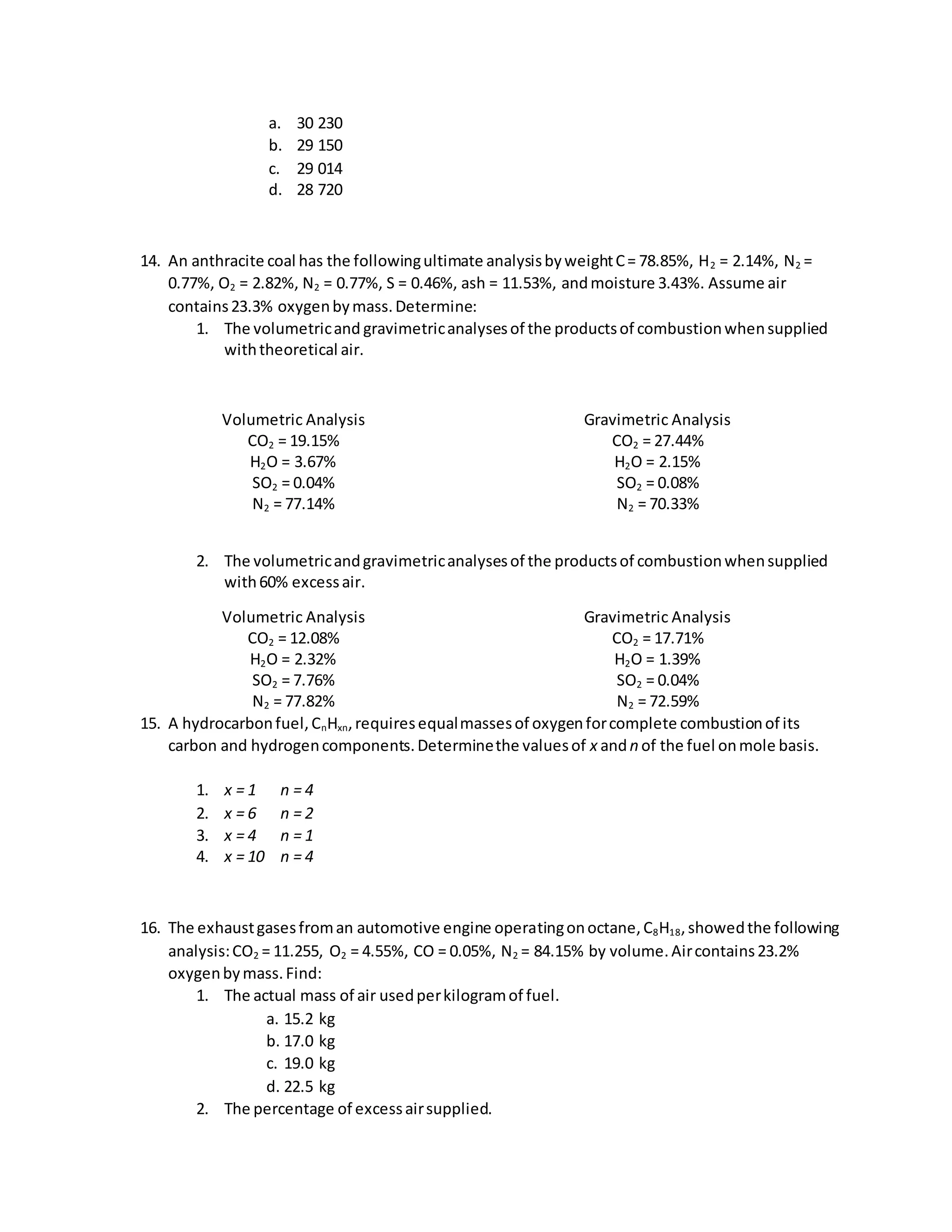
This document provides information about the composition and combustion analysis of various fuels including coal, natural gas, and ethanol. It gives the ultimate and proximate analyses, on different bases, of different coal and fuel samples. It also provides calculations for determining theoretical air requirements, excess air, heating values, combustion product compositions and volumes.