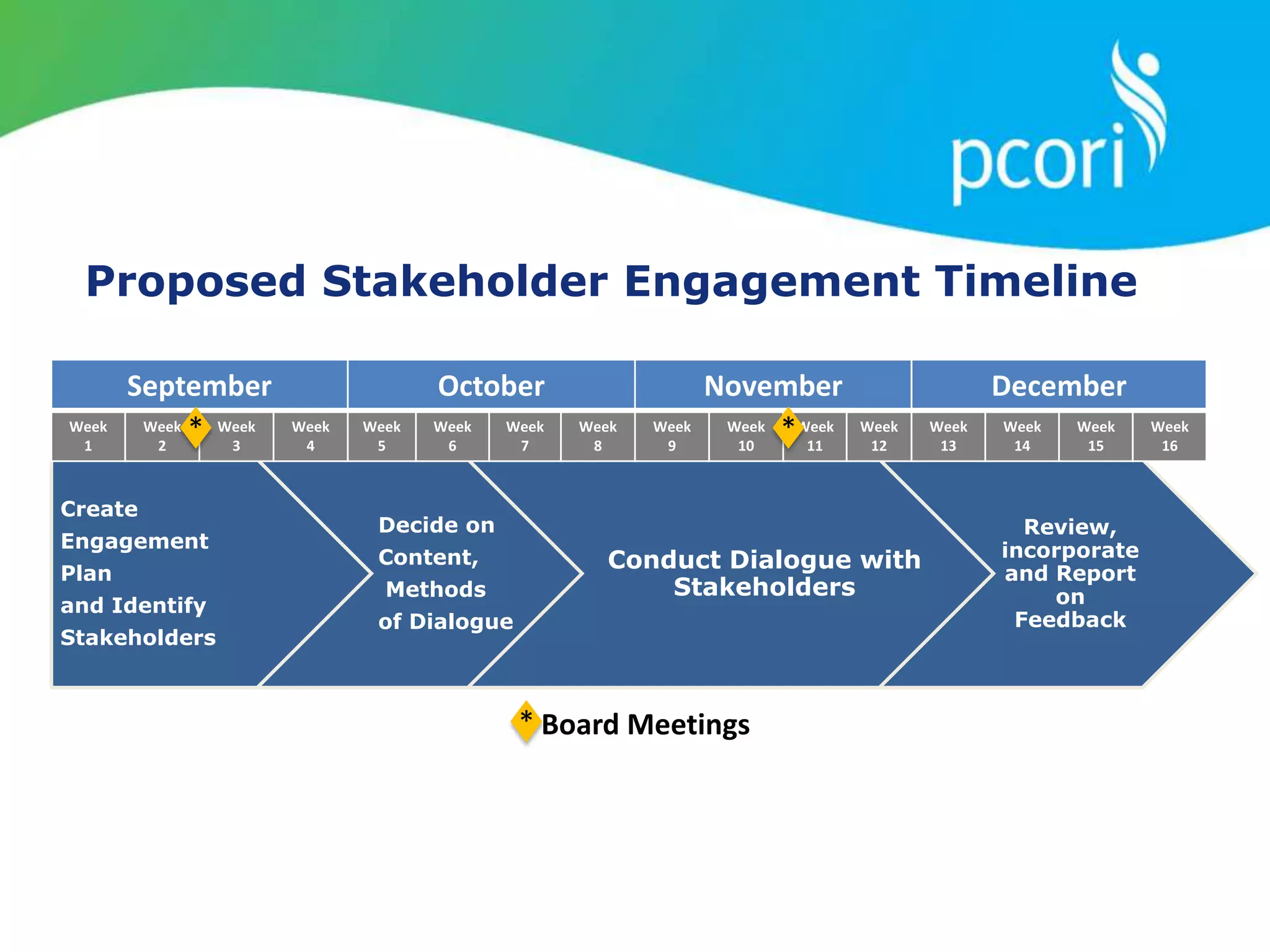
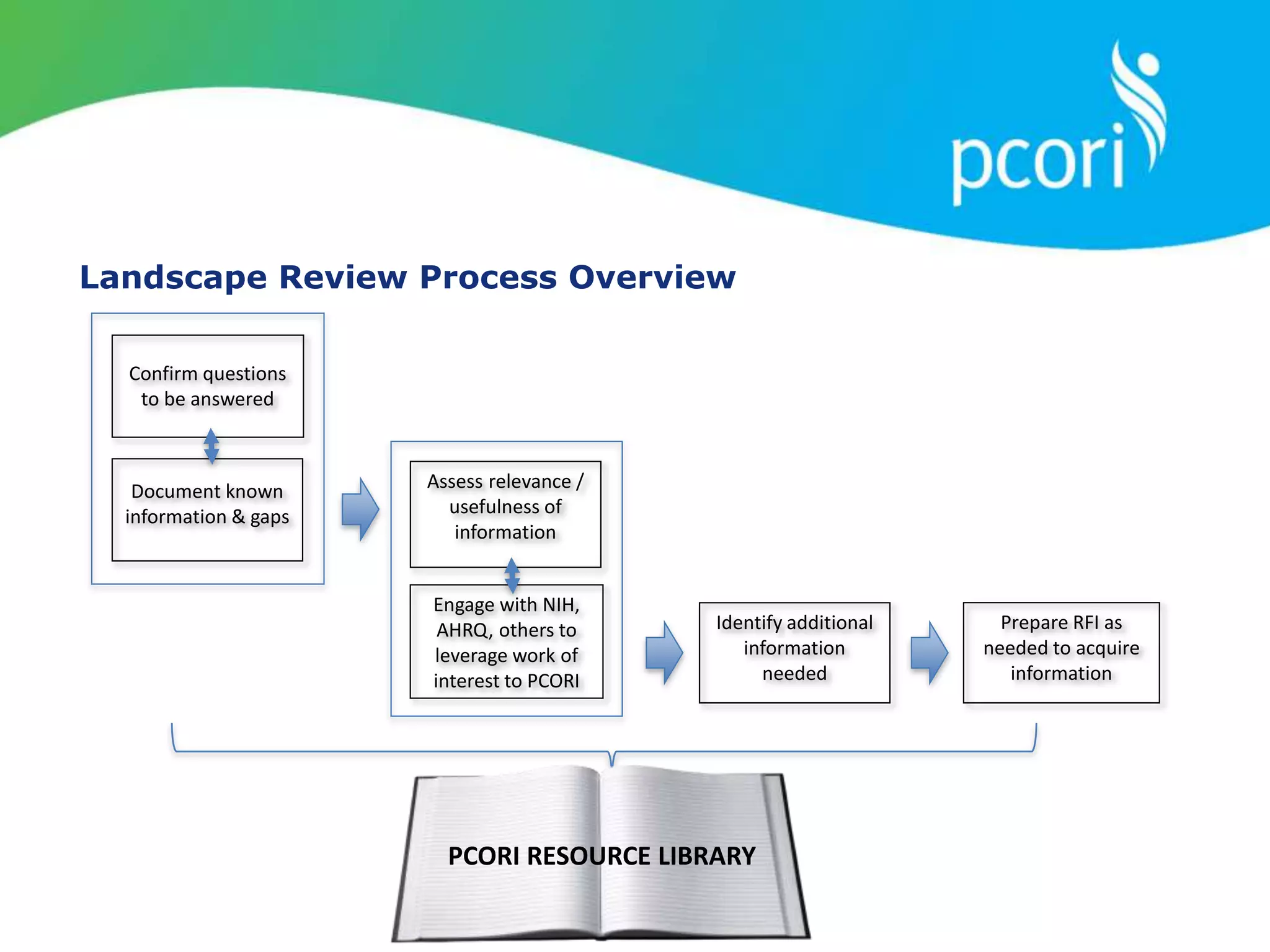
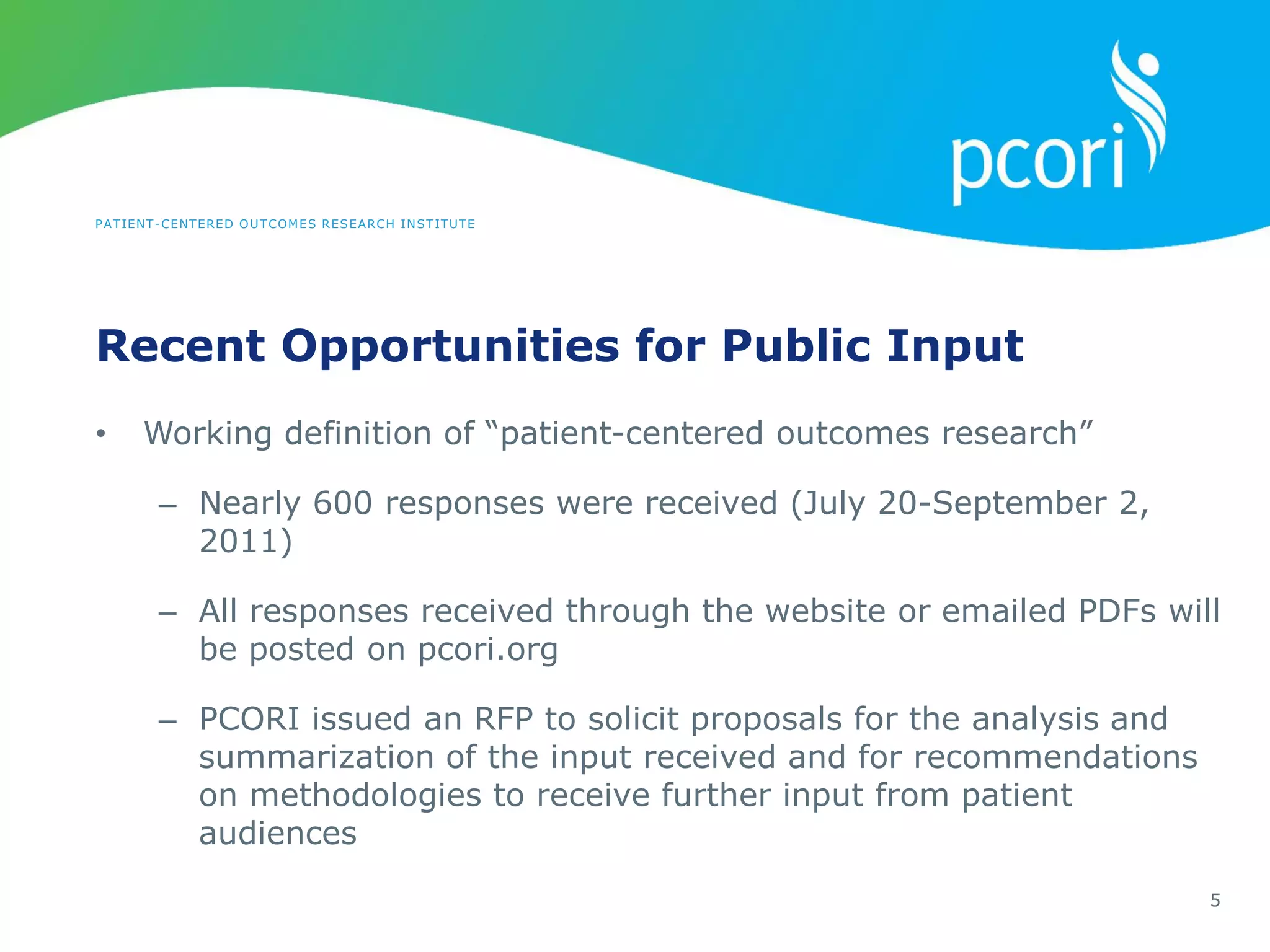
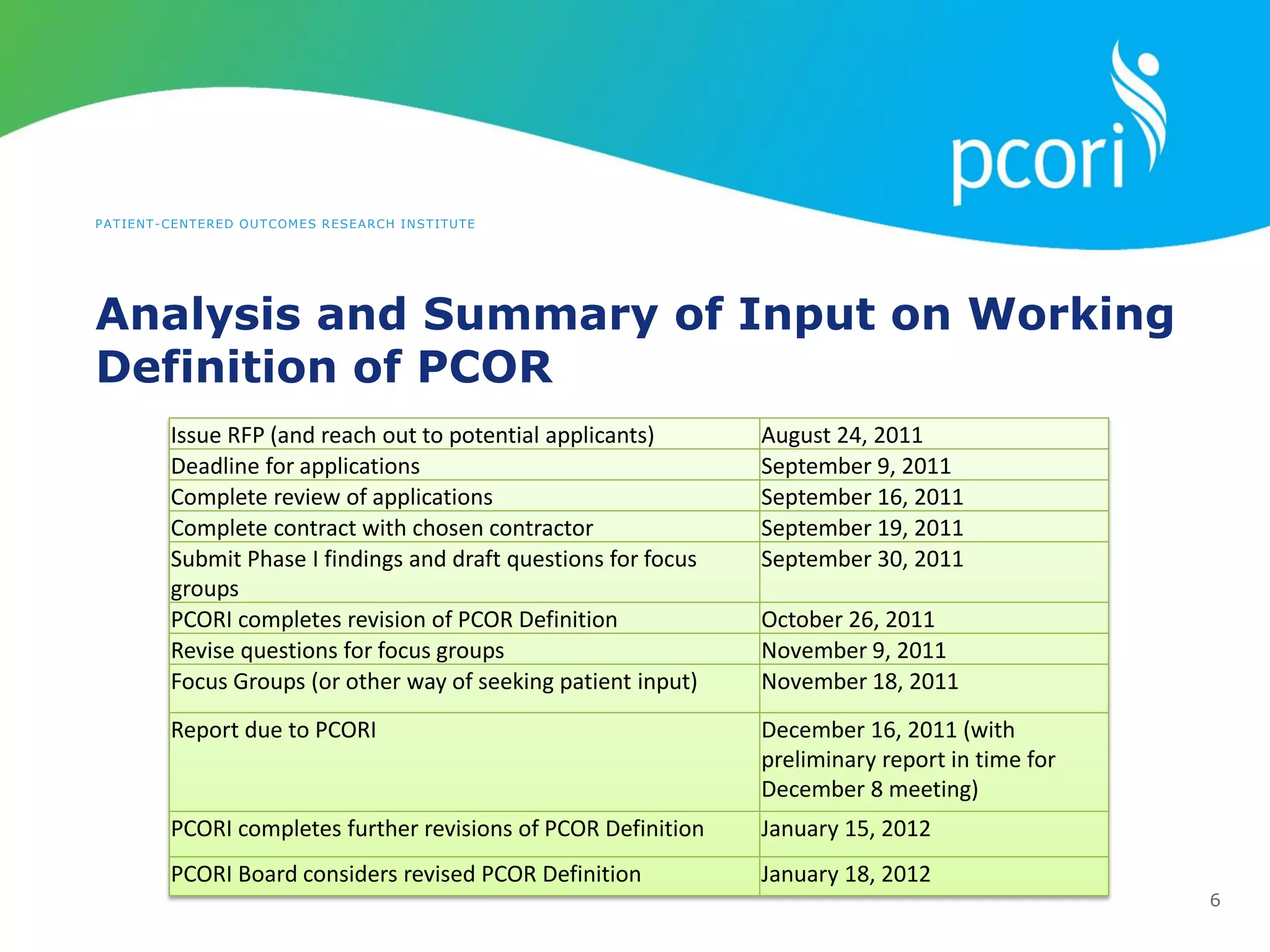
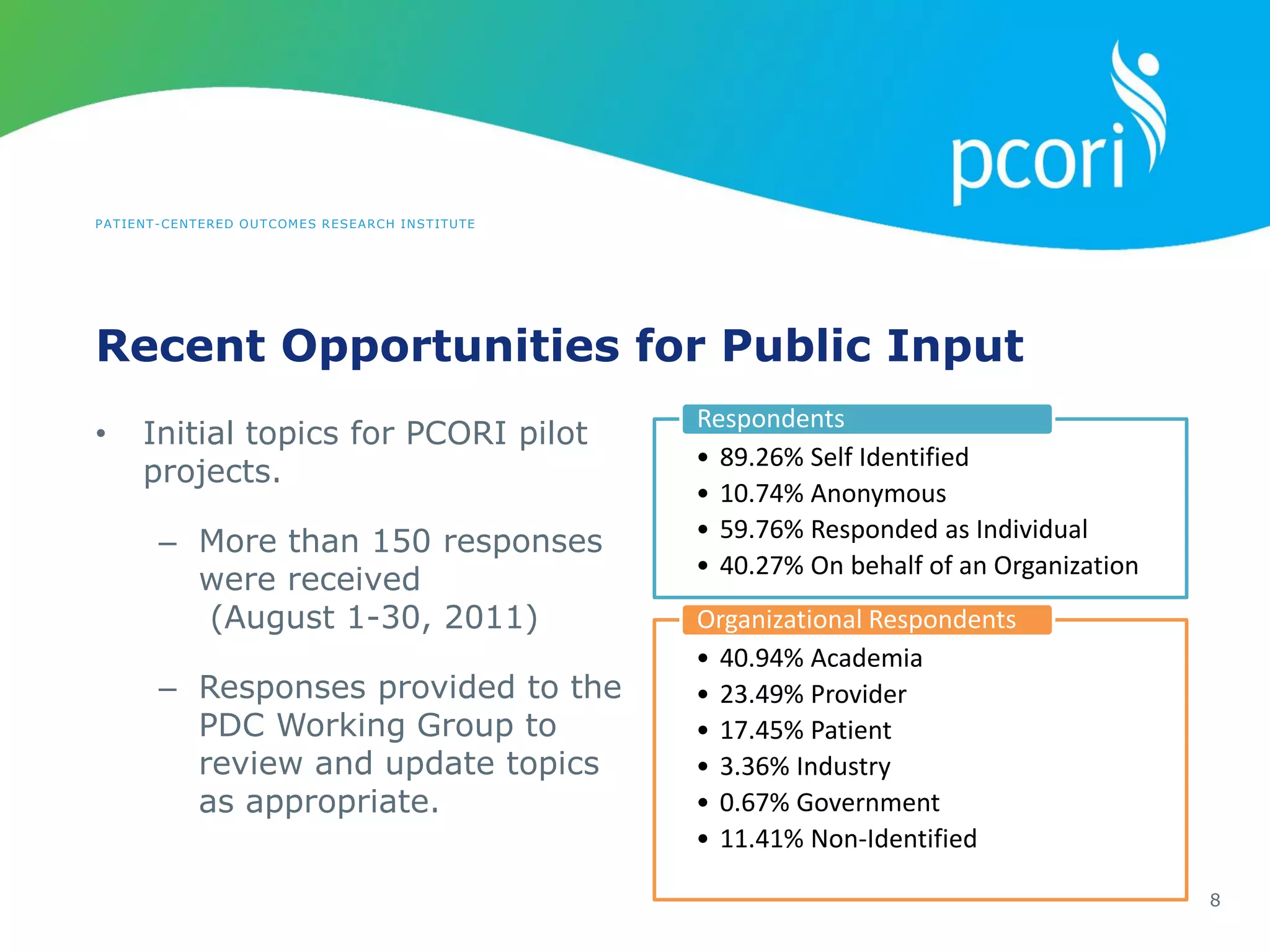
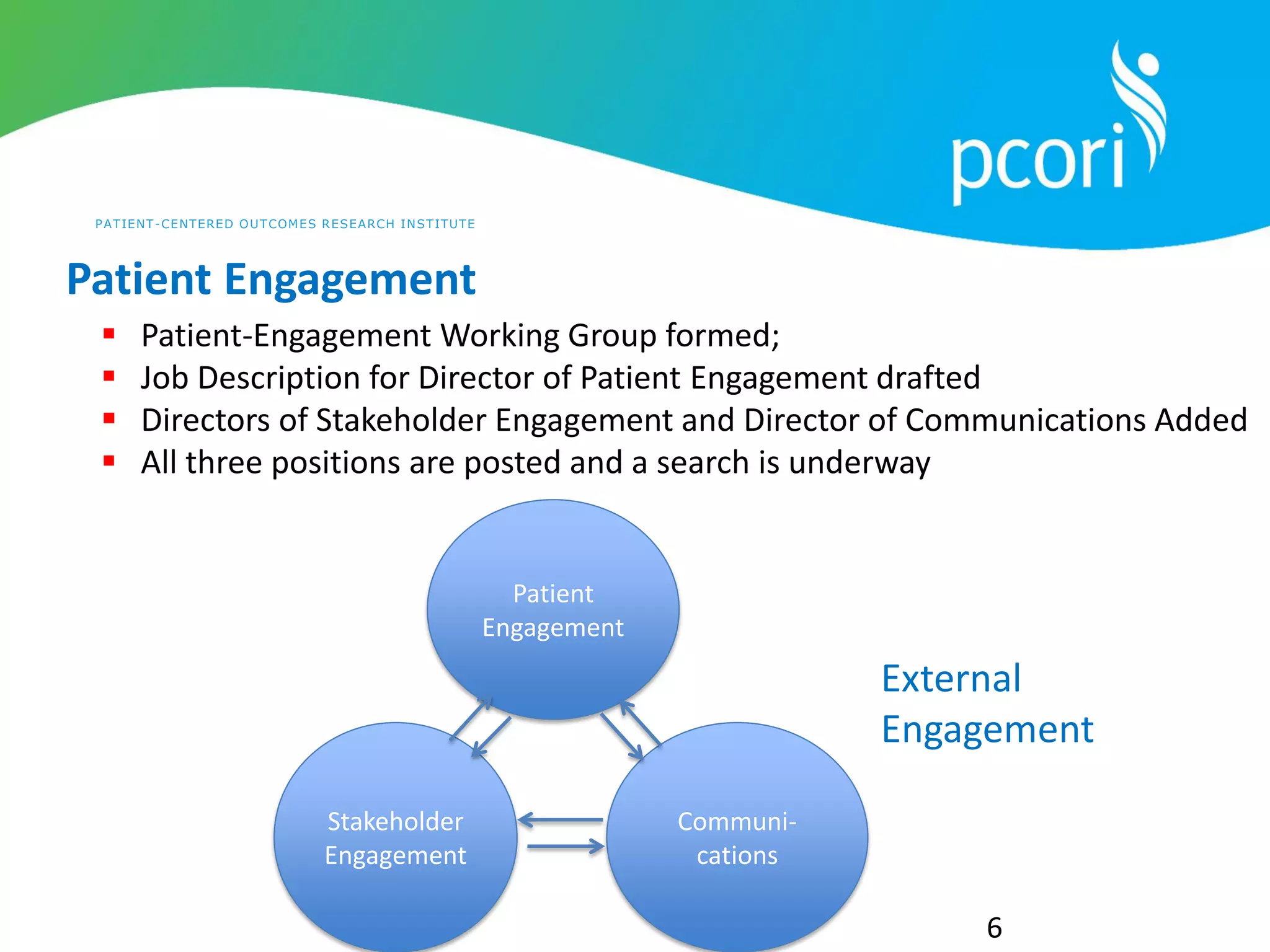
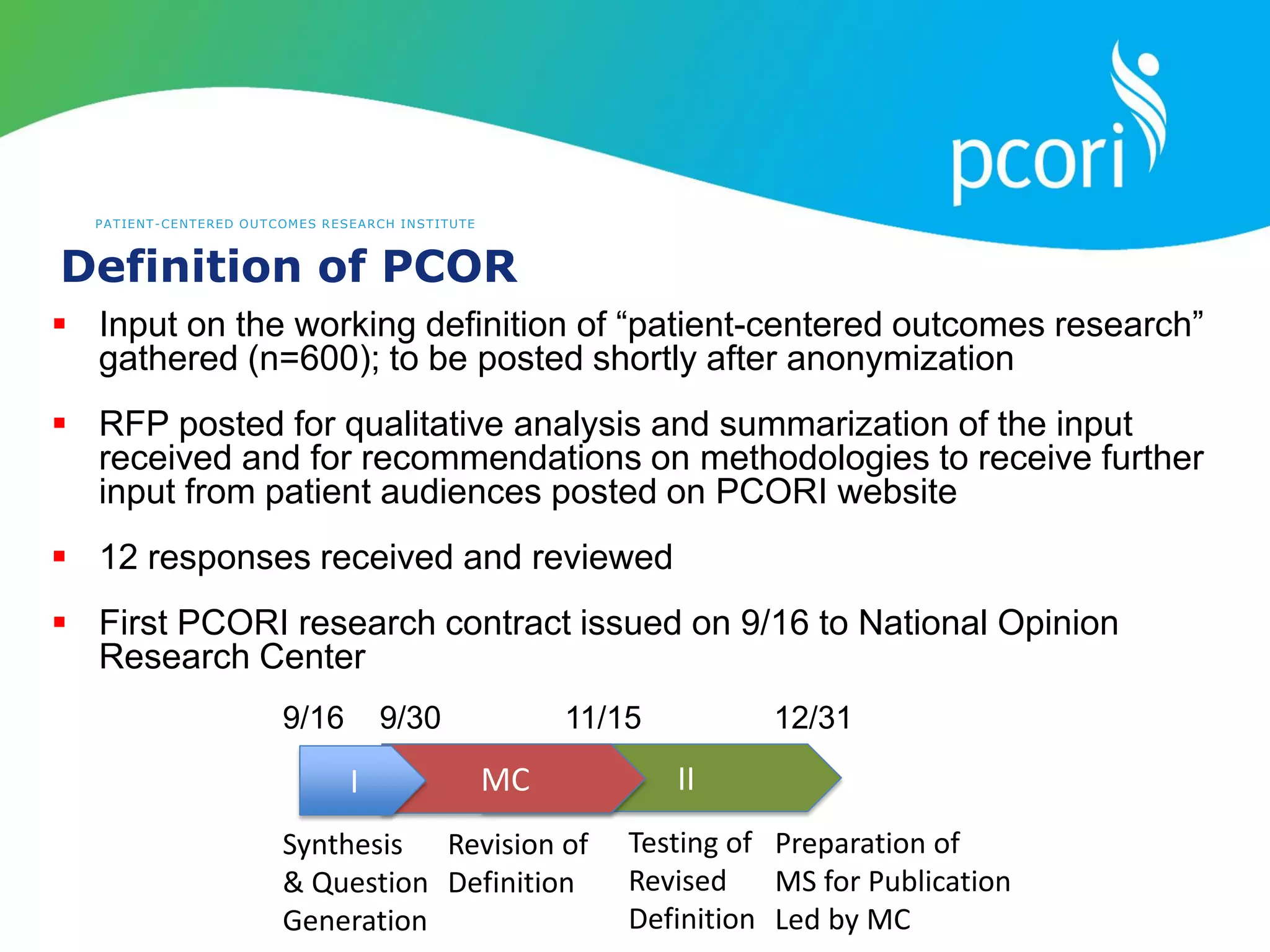
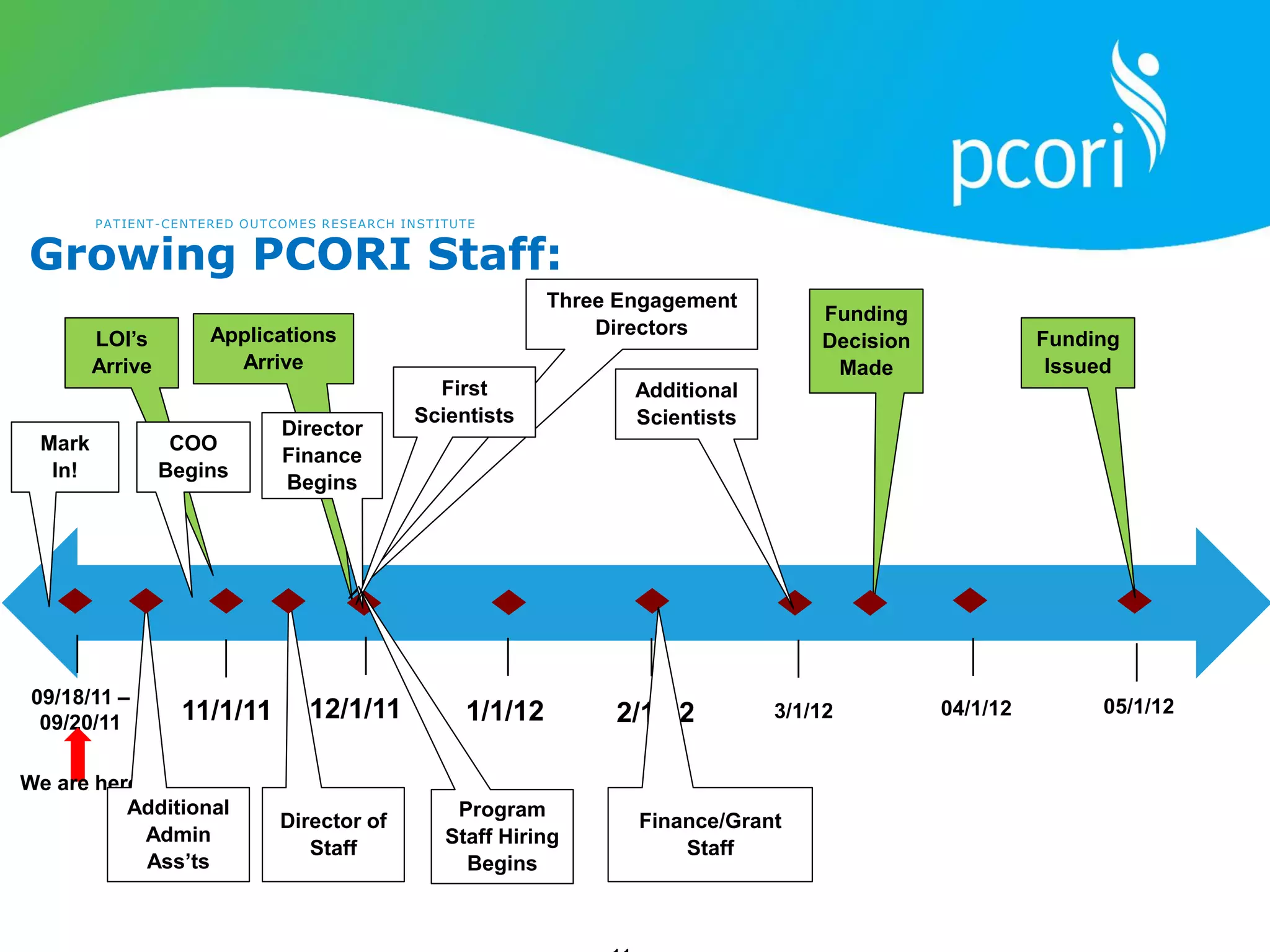
This document provides a summary of the September 19, 2011 report from the Communications, Outreach and Engagement Committee (COEC) of the Patient-Centered Outcomes Research Institute (PCORI). The summary includes updates on recent public input opportunities, promoting PCORI funding opportunities, redesigning the PCORI website, outreach plans, and growing the PCORI communications staff.


































![PATIENT-CENTERED OUTCOMES RESEARCH INSTITUTE
14
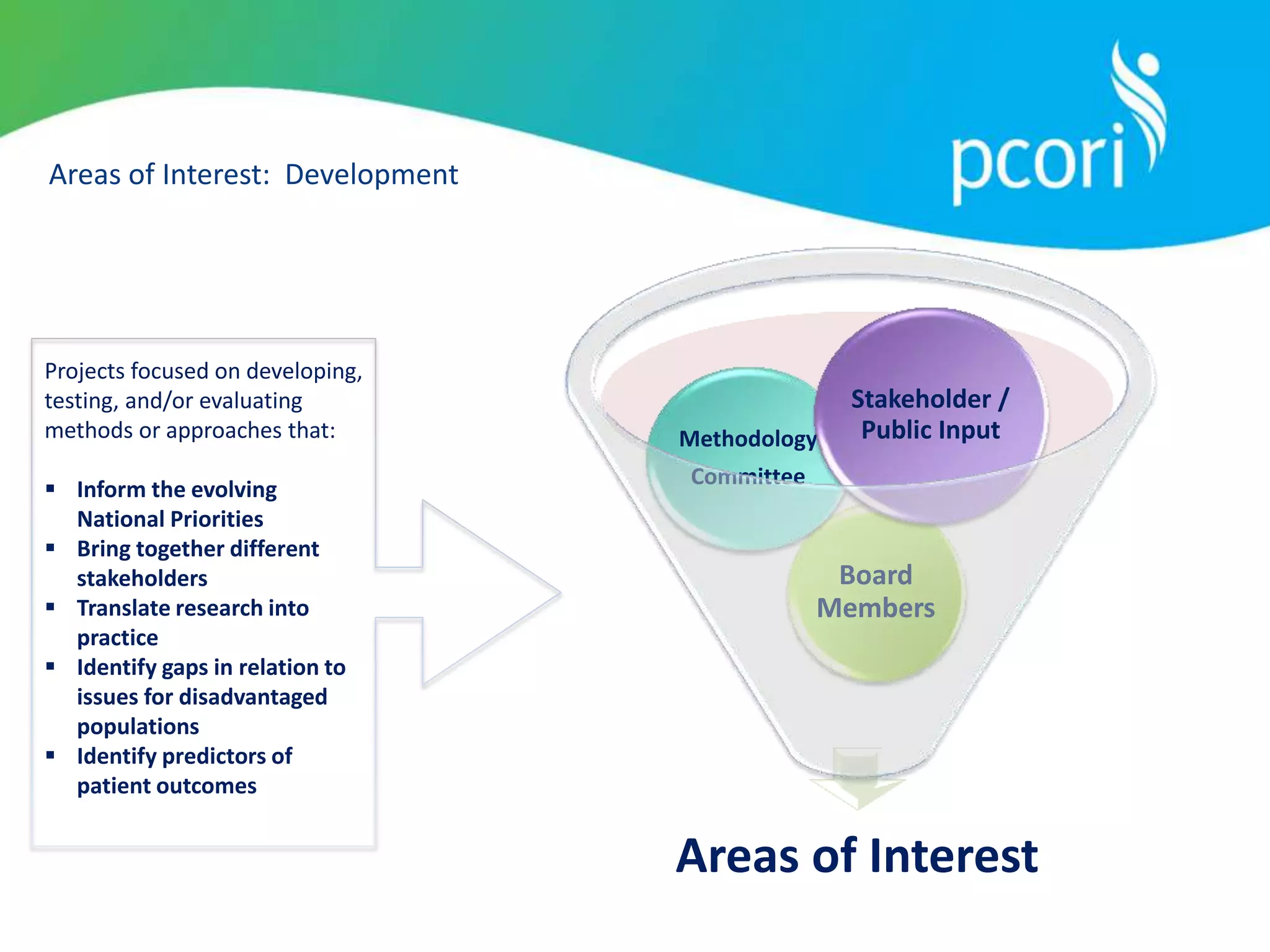
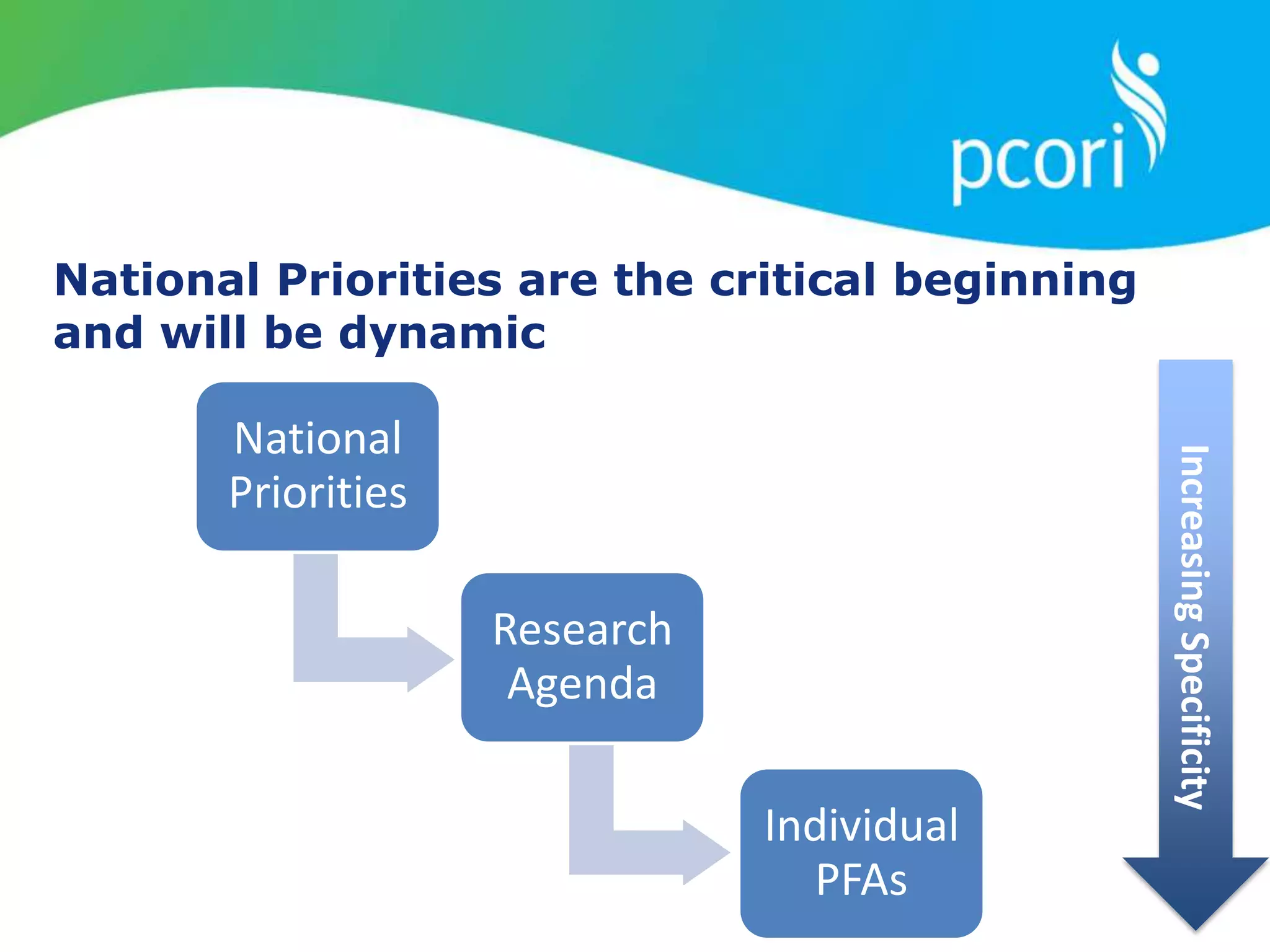
Research Prioritization Mission
Mission
To provide guidance concerning the use of methods to inform the
establishment of research prioritization approaches that best
fulfill PCORI’s mission.
• To the PCORI Board to aid in development and future
refinement of Research Priorities]
• To the broader multi-stakeholder communities to enhance
understanding of PCORI’s approach to research prioritization
and to encourage their engagement in PCORI’s research
prioritization process](https://image.slidesharecdn.com/combinedpowerpoints-130805105954-phpapp01/75/Board-of-Governors-Meeting-South-Sea-Tea-Washington-49-2048.jpg)