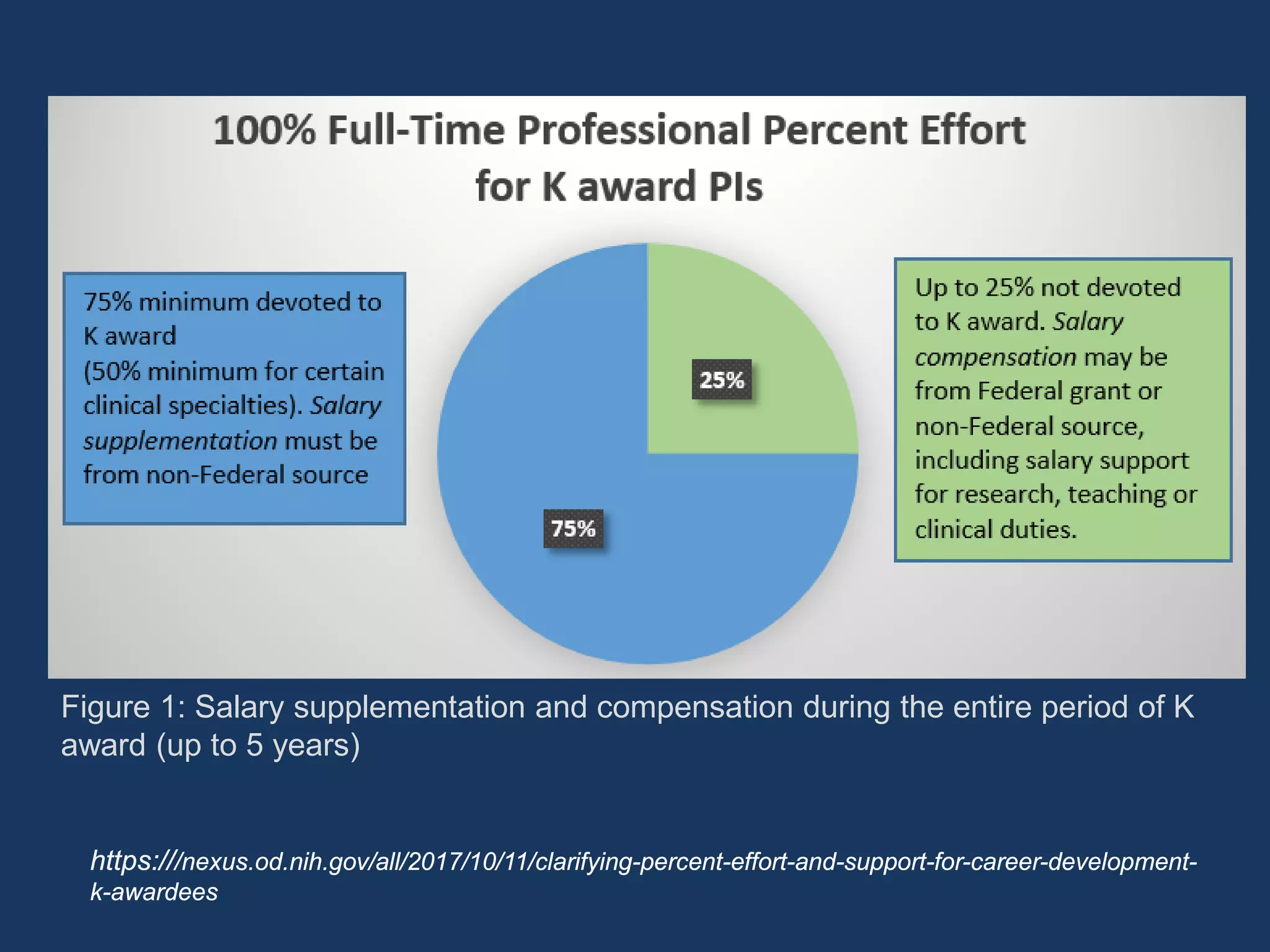
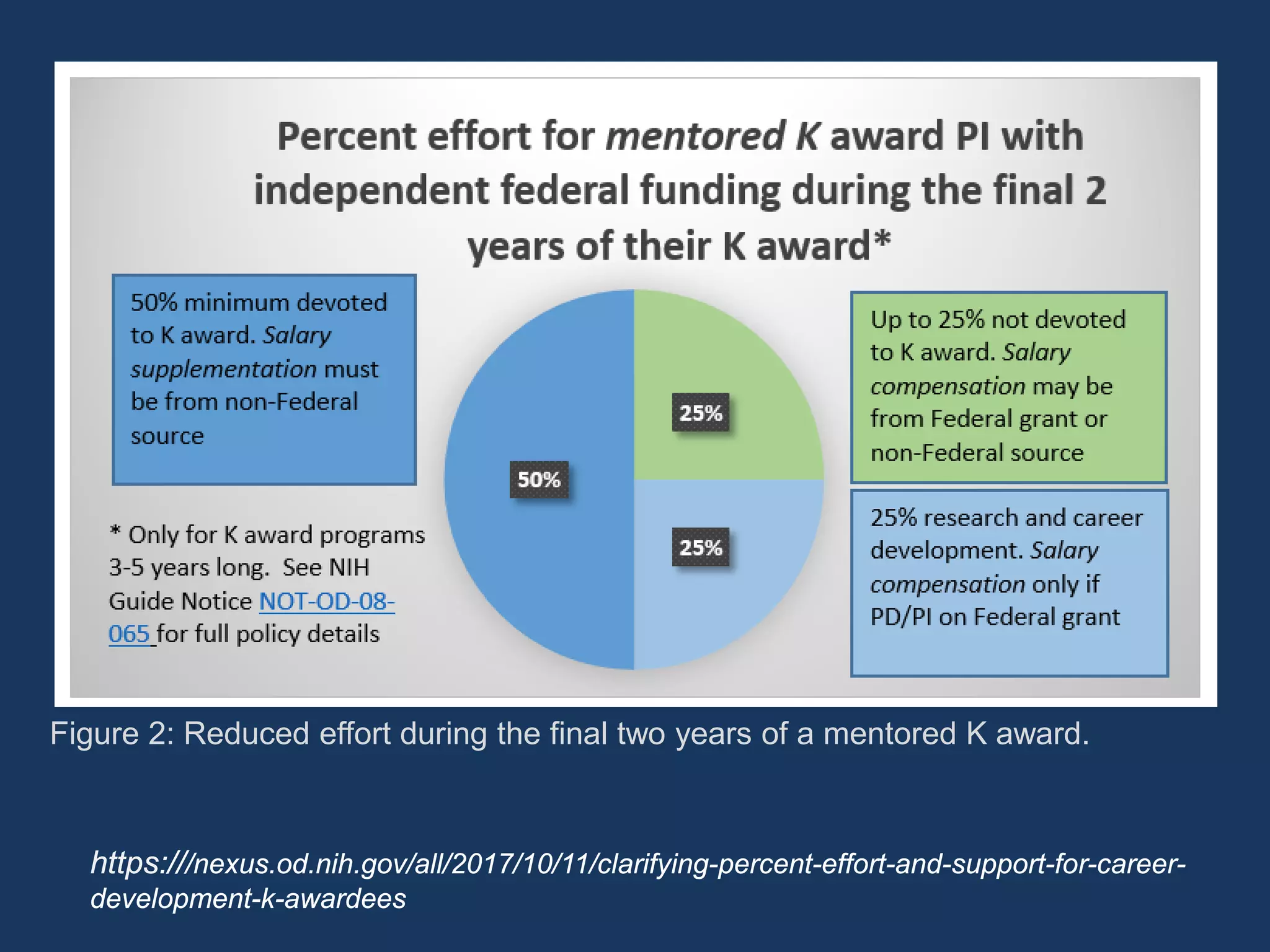
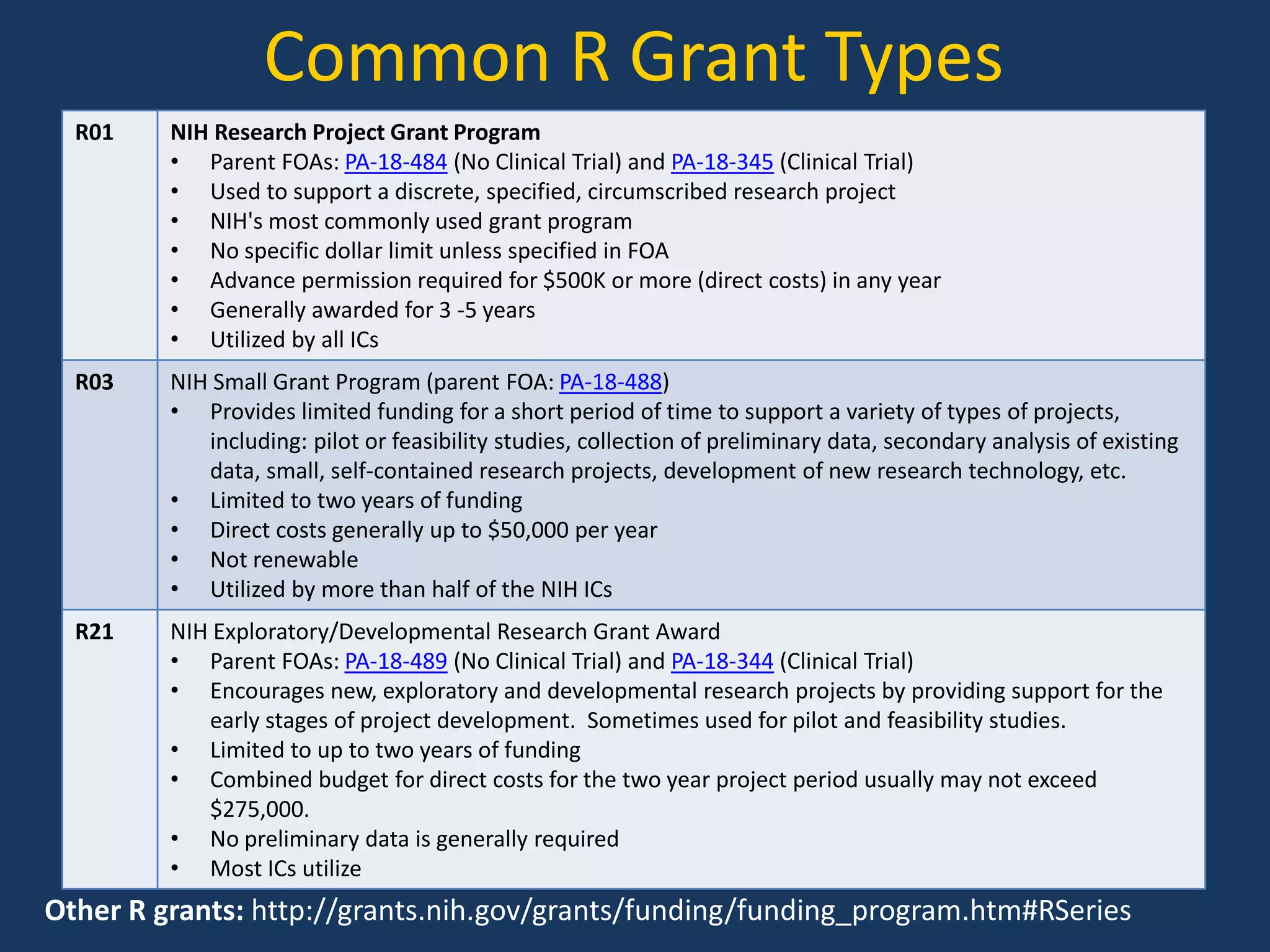
The document provides guidance on preparing for an R grant application, emphasizing the significance of building relationships with NIH program officers and being proactive in research visibility. It outlines strategies for utilizing K award research effectively, including identifying key research questions and seeking opportunities for independence. Additionally, it discusses funding transition from K to R grants, emphasizing the importance of understanding funding mechanisms and developing a strong research proposal.