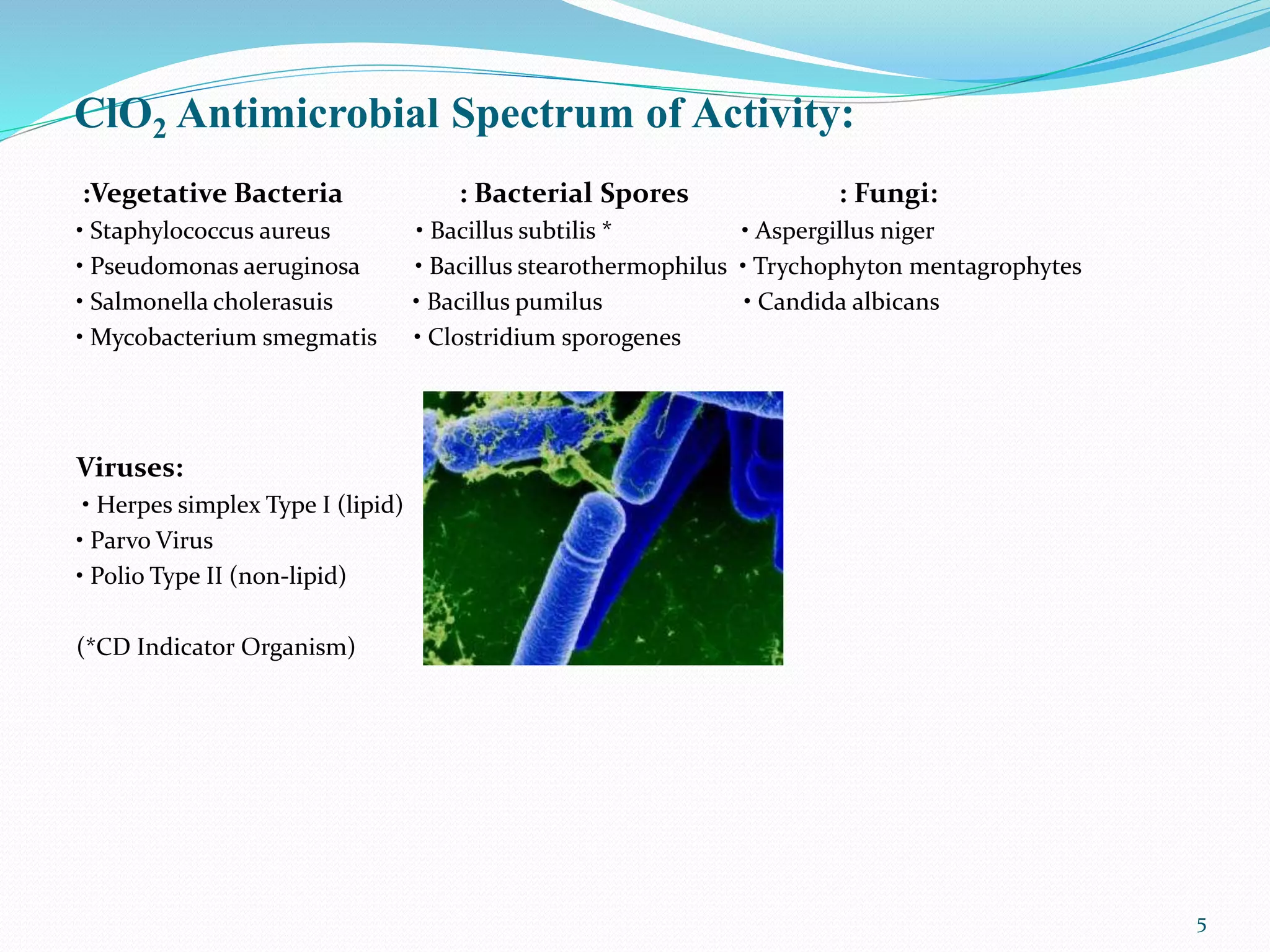
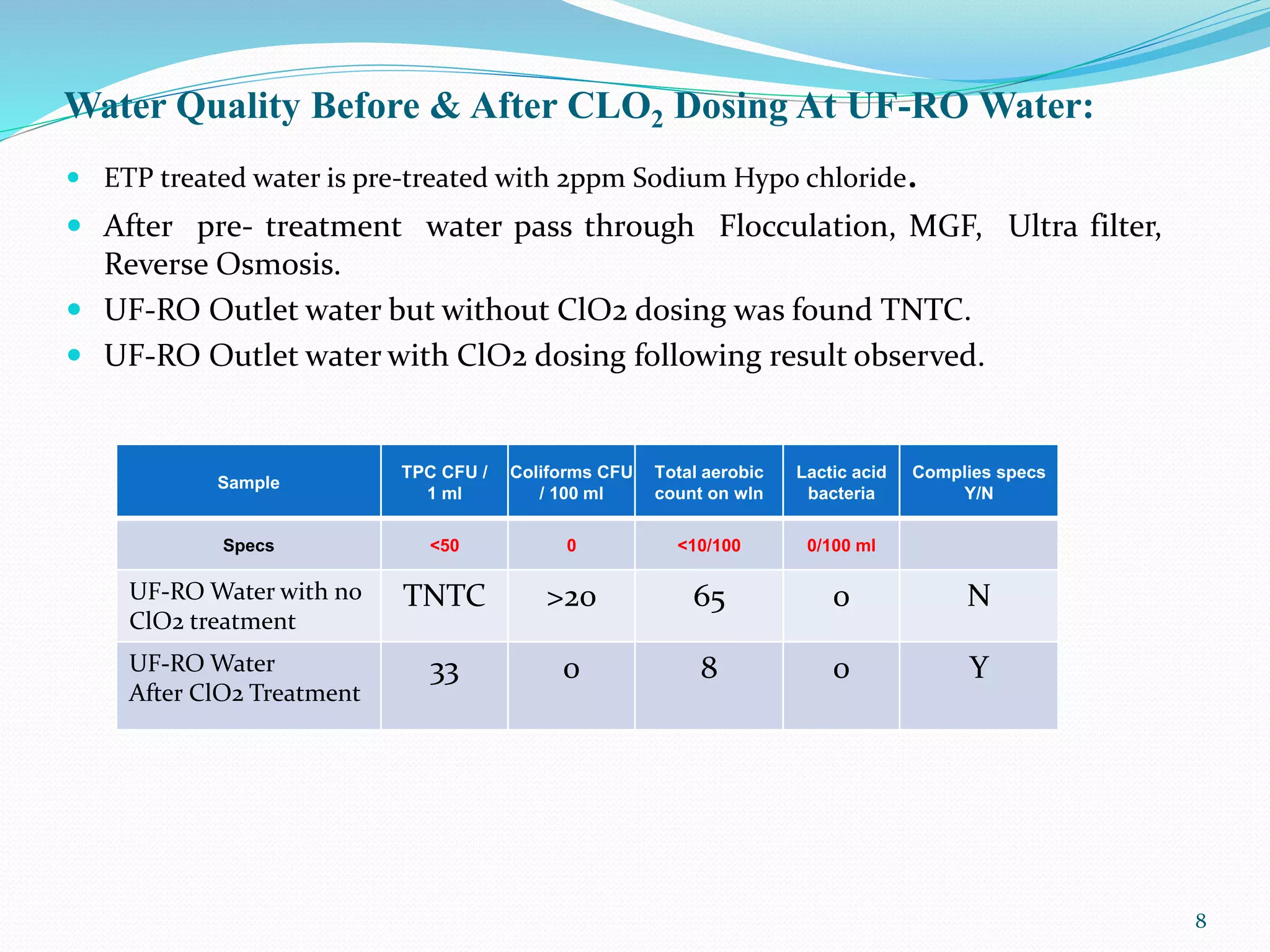
The document outlines the properties and applications of chlorine dioxide (ClO2) as an antimicrobial agent used in water treatment processes, emphasizing its effectiveness against various pathogens. It details the operational procedures for ClO2 dosing in water treatment facilities, including installation specifics and safety guidelines for handling the chemical. Additionally, the document provides data on water quality improvements achieved through ClO2 treatment compared to untreated water.