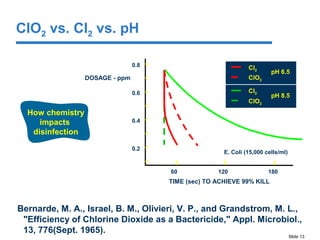
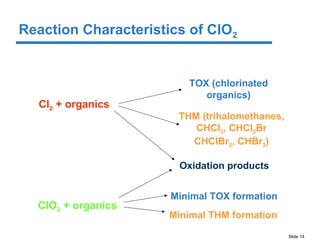
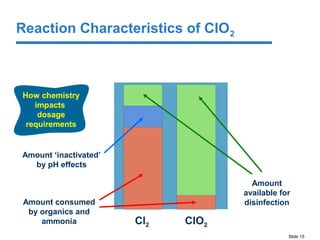
This document discusses the effectiveness of chlorine (Cl2) and chlorine dioxide (ClO2) for controlling biofilms. It summarizes literature findings that Cl2 does not penetrate or remove biofilms well, while ClO2 is more effective at penetrating and removing biofilms. ClO2 is also less affected by pH and forms fewer disinfection byproducts than Cl2. However, ClO2 has some disadvantages in that it must be made on-site using a generator and multiple precursors, which can increase costs compared to Cl2 in some situations.