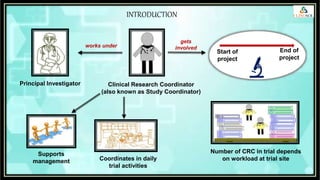
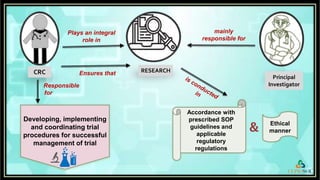
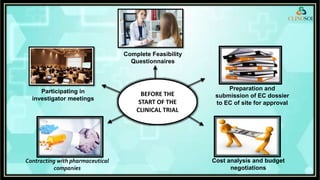
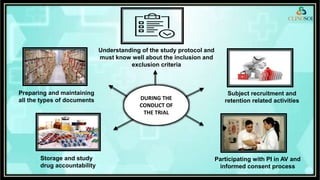
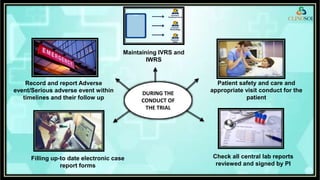
The document outlines the role of a Clinical Research Coordinator (CRC) in managing clinical trials, detailing their responsibilities from start to finish. Key duties include supporting the Principal Investigator, coordinating trial procedures, maintaining documentation, and ensuring patient safety. Additionally, the CRC is responsible for document verification and archiving following the trial's close.