Embed presentation
Download to read offline
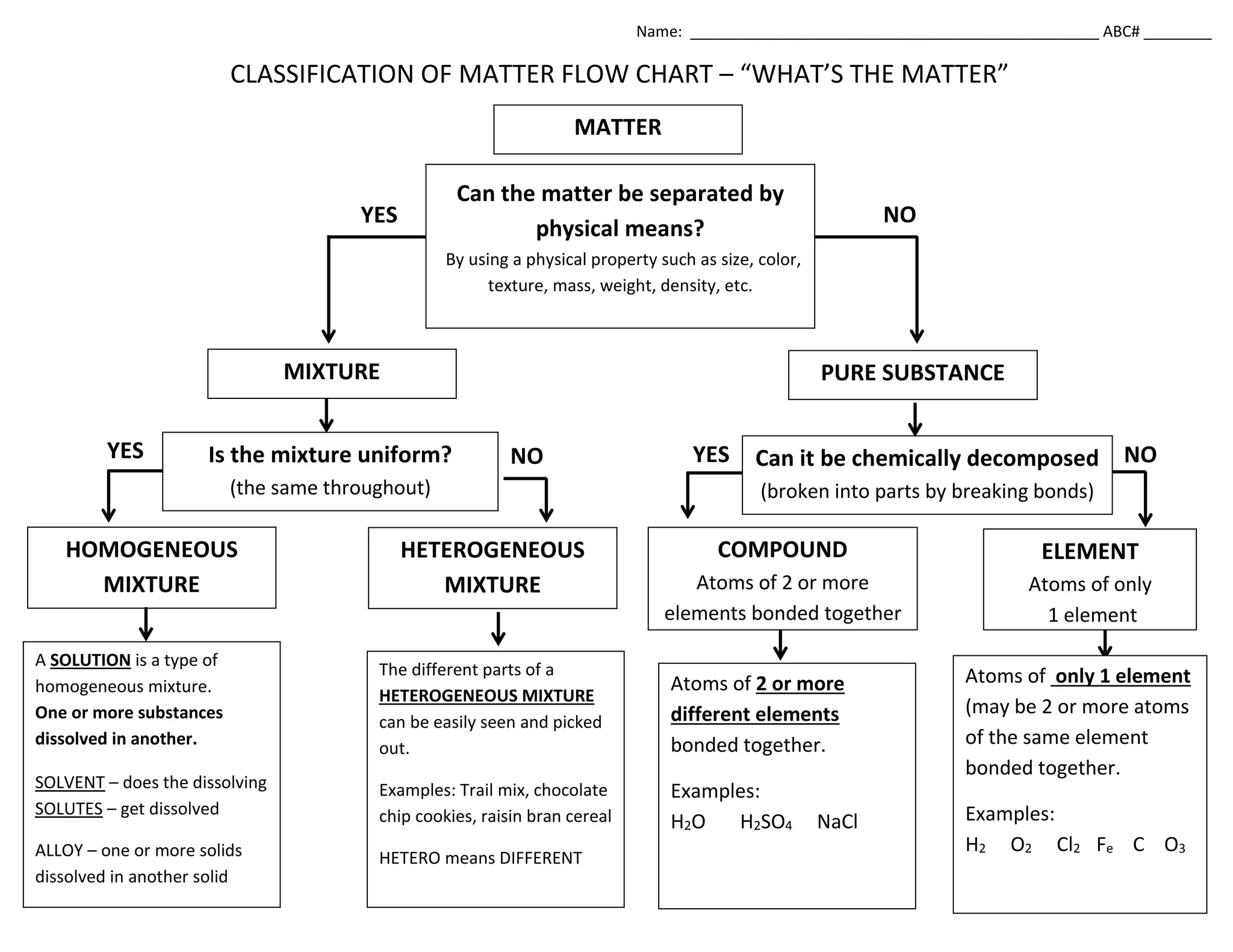
This flow chart classifies matter based on its composition and ability to be separated. It can be summarized as: 1) Matter is either a pure substance that cannot be chemically decomposed, or a mixture that can be separated into parts. 2) Pure substances are either elements made of one type of atom or compounds made of two or more types of atoms bonded together. 3) Mixtures are either homogeneous, meaning uniform throughout, or heterogeneous, where the parts can be easily seen and separated. Common homogeneous mixtures are solutions and alloys.
