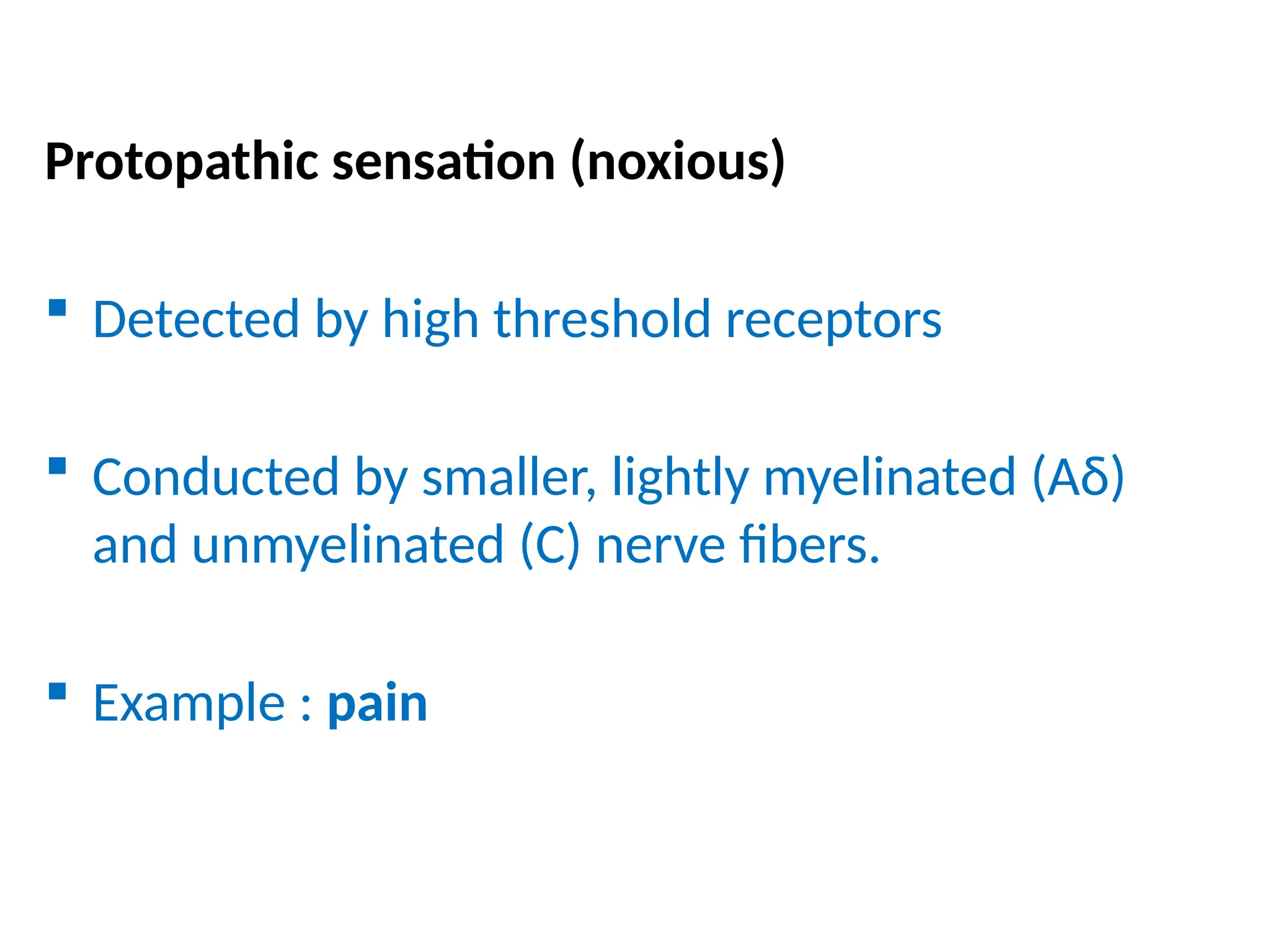
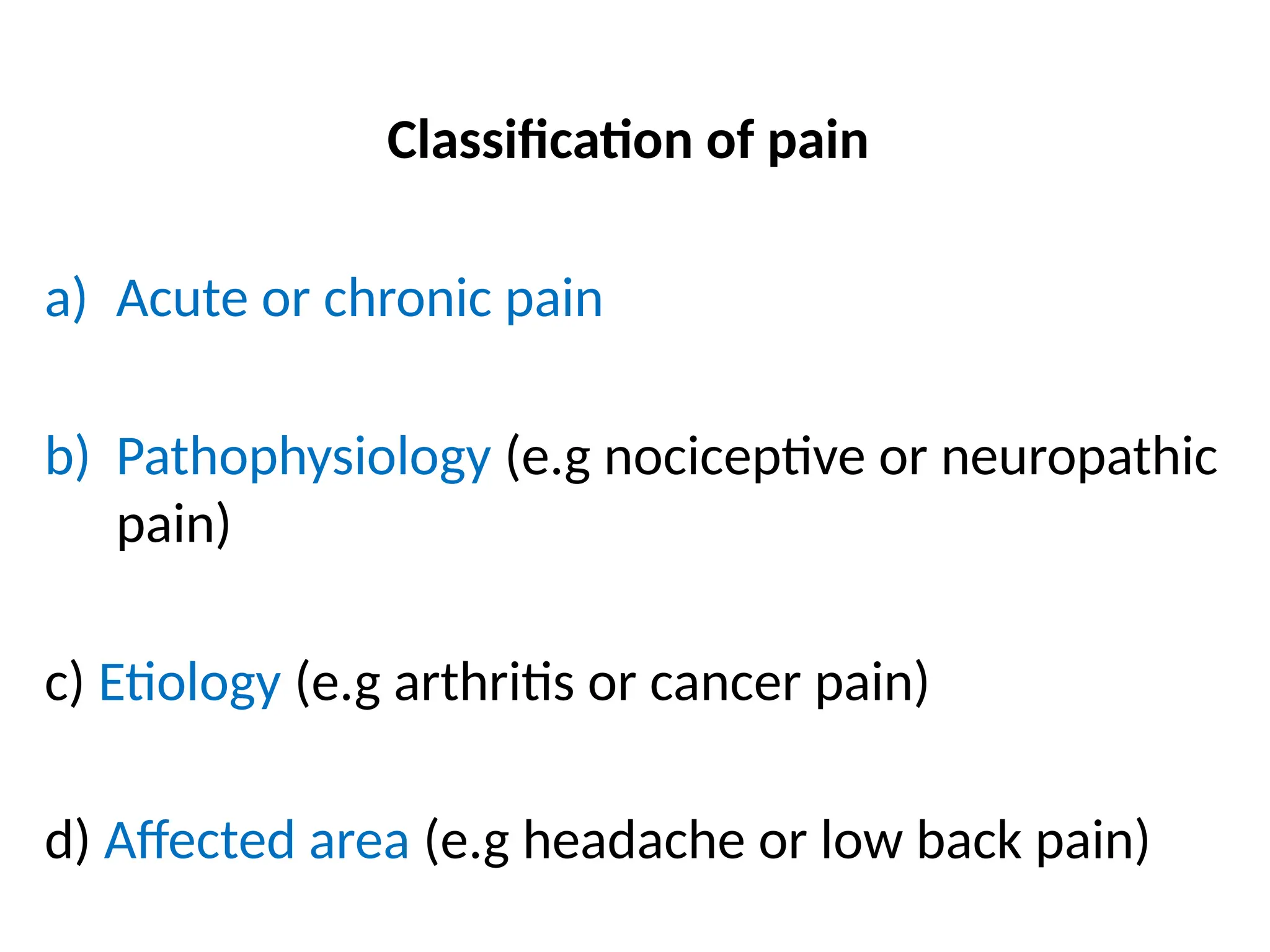
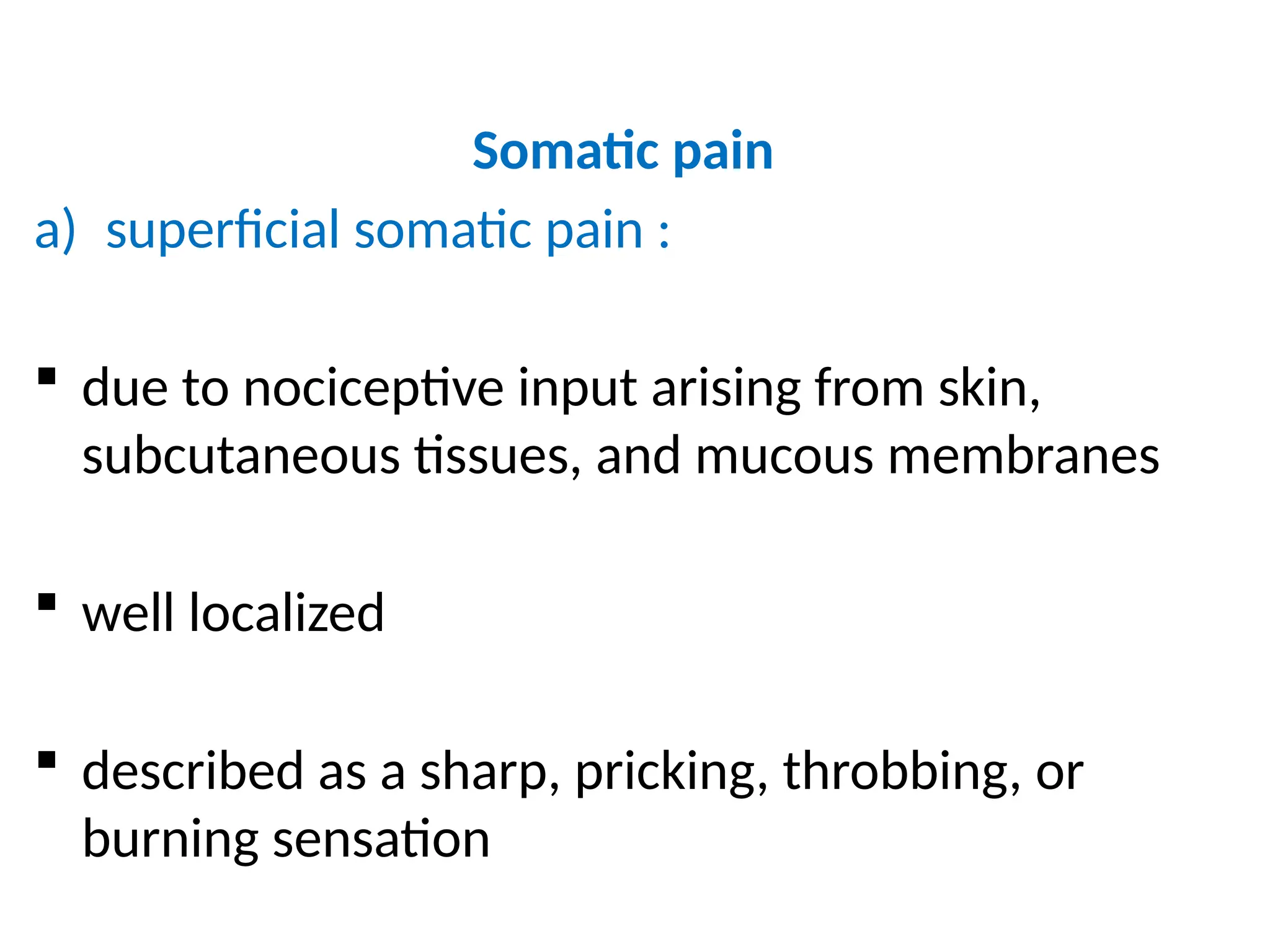
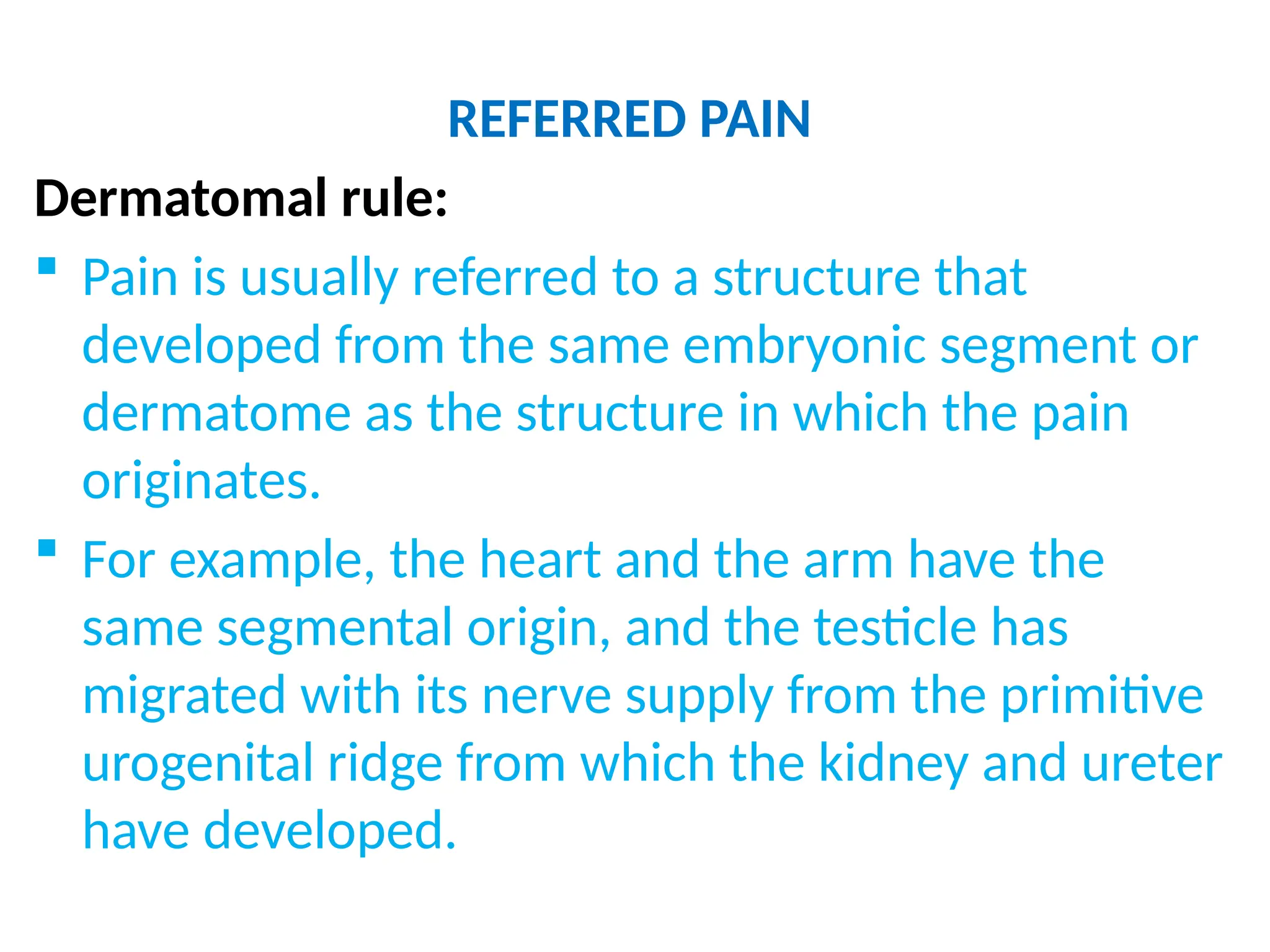
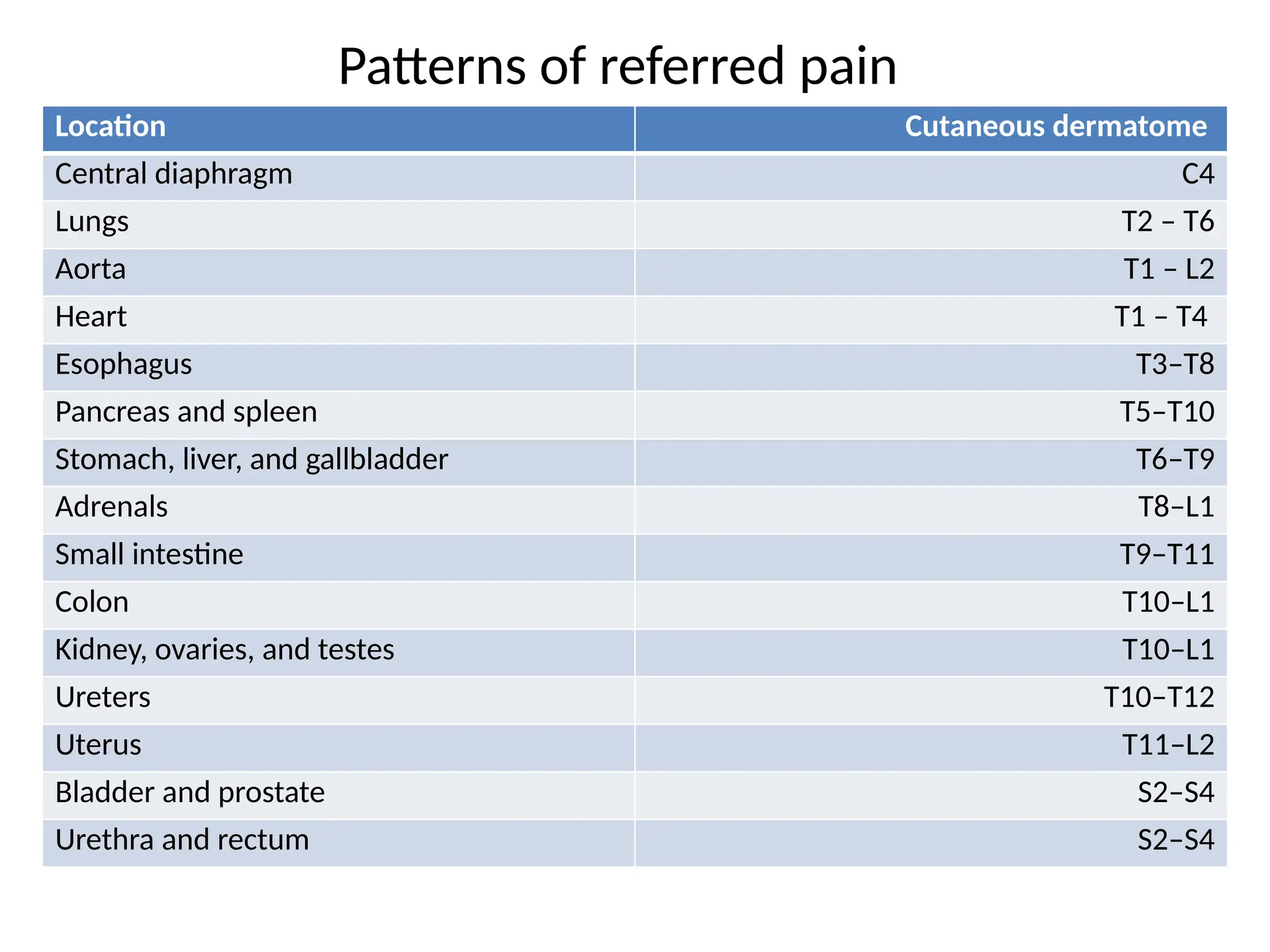
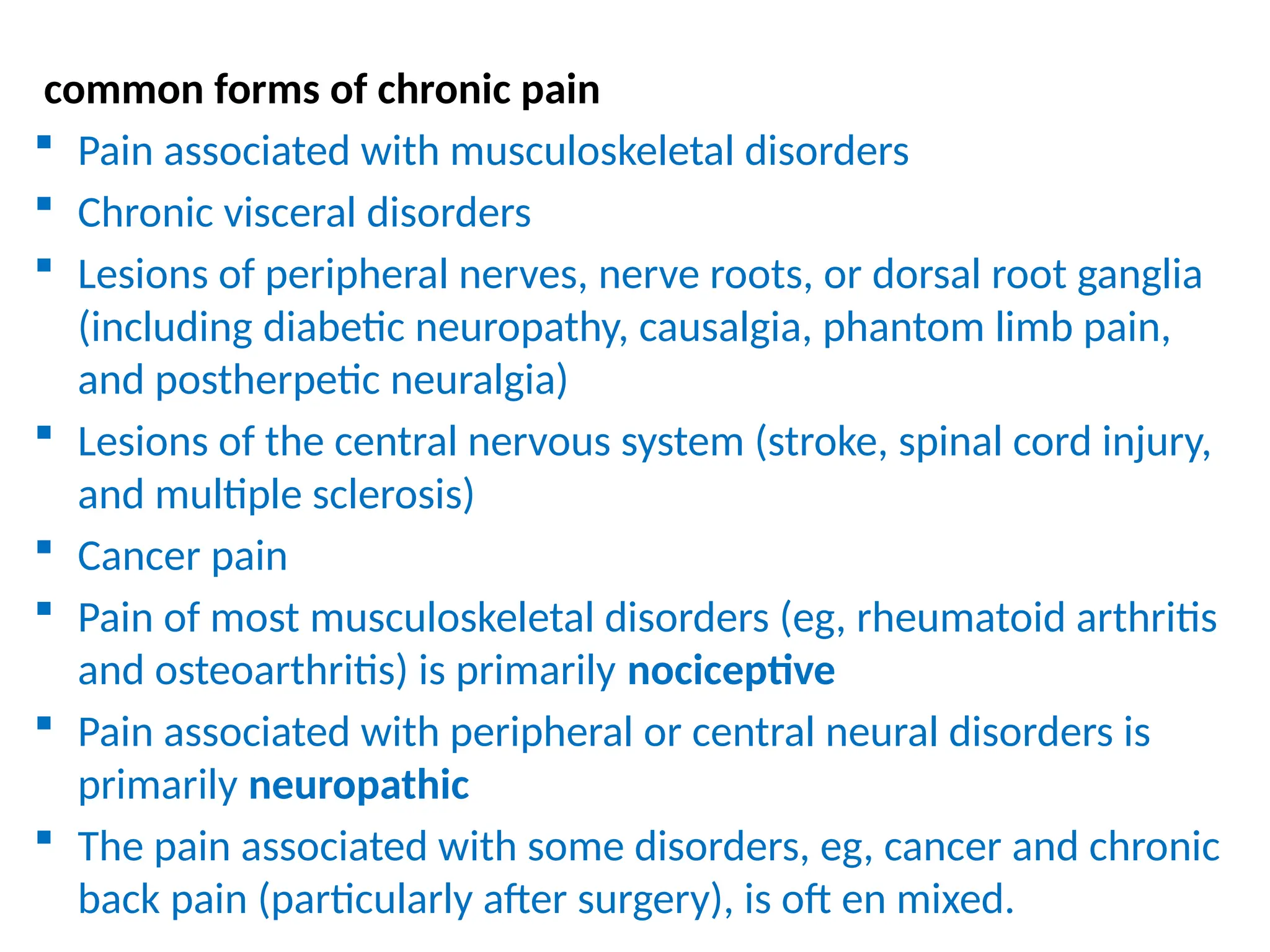
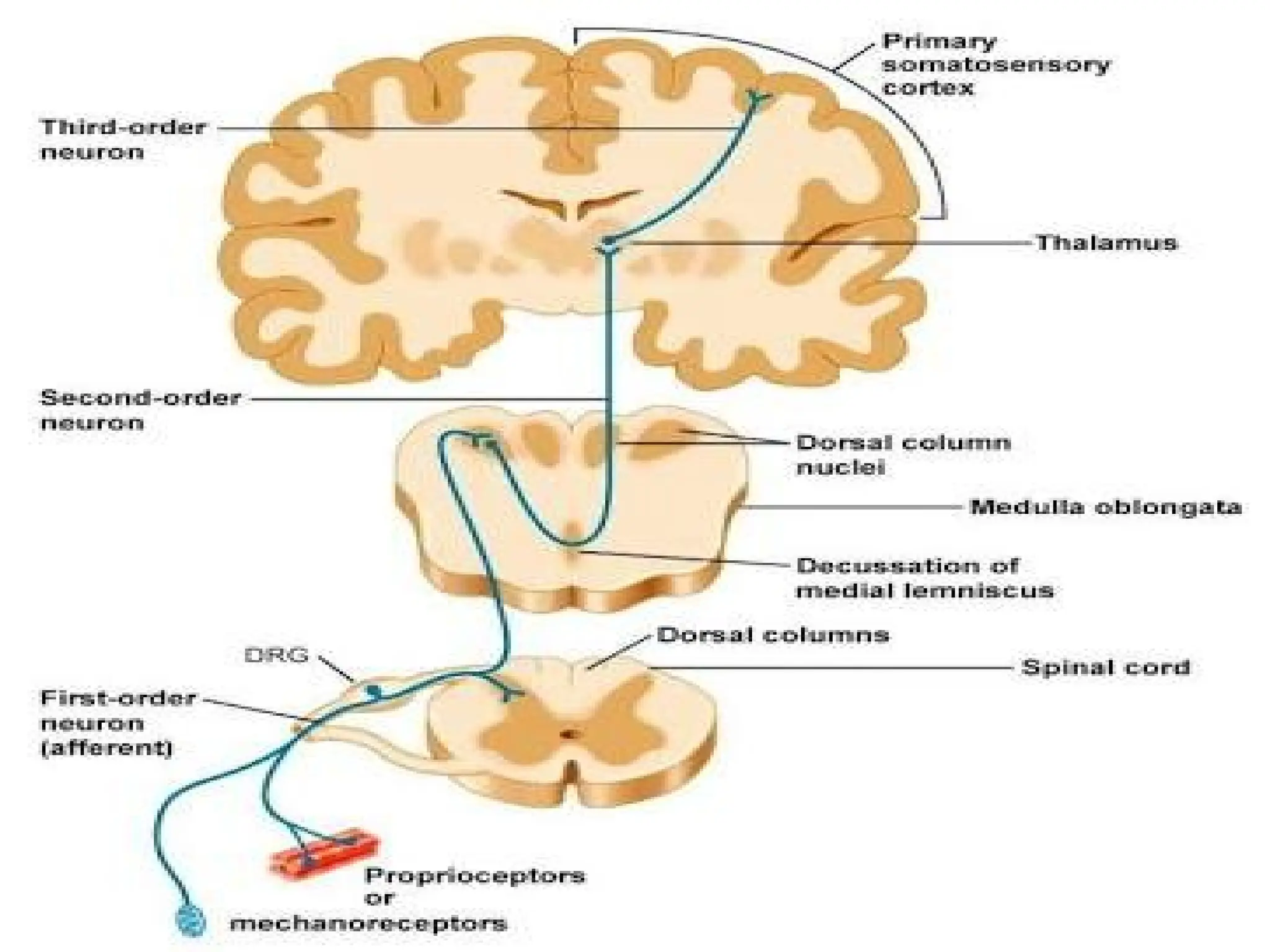
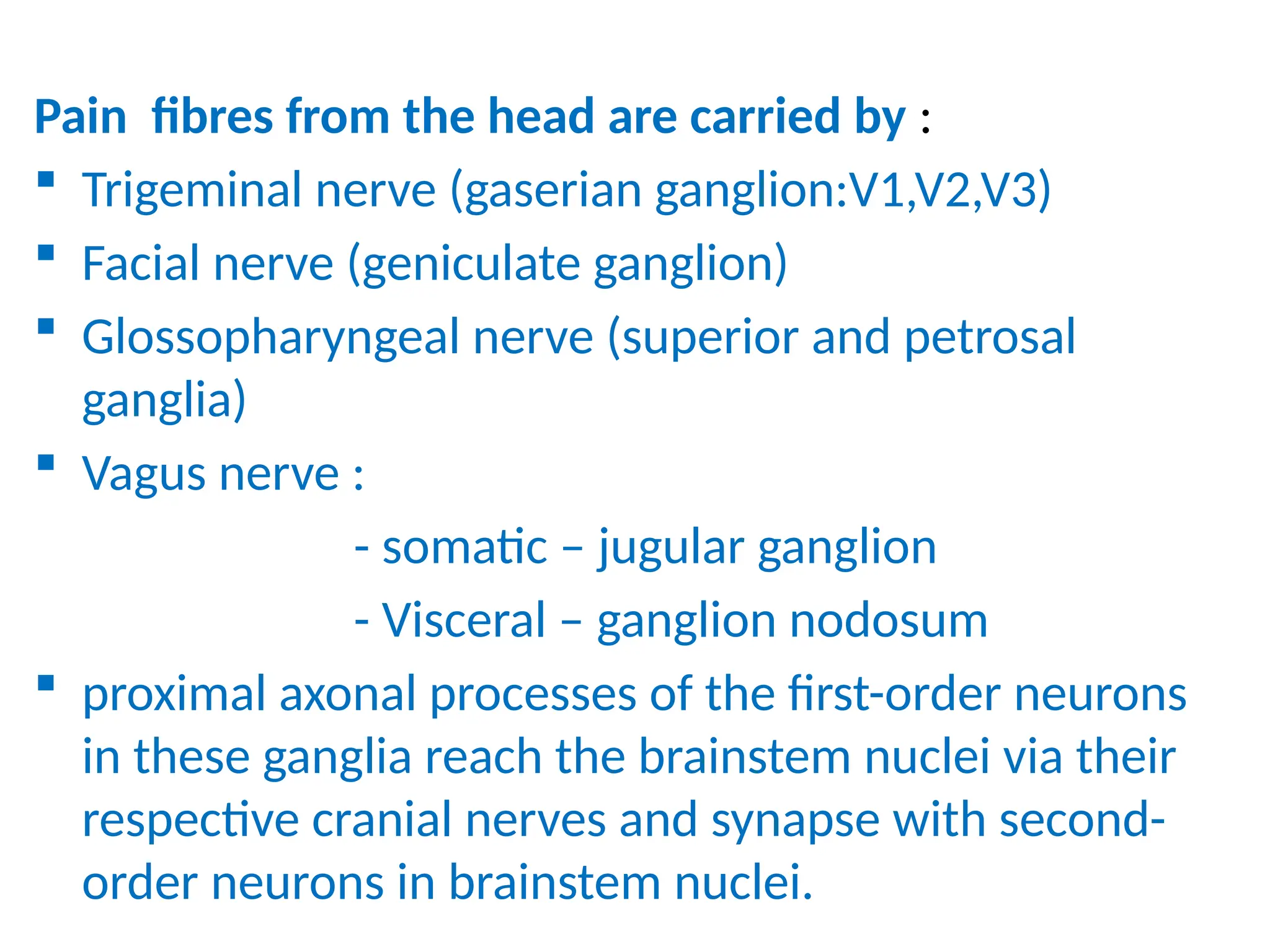
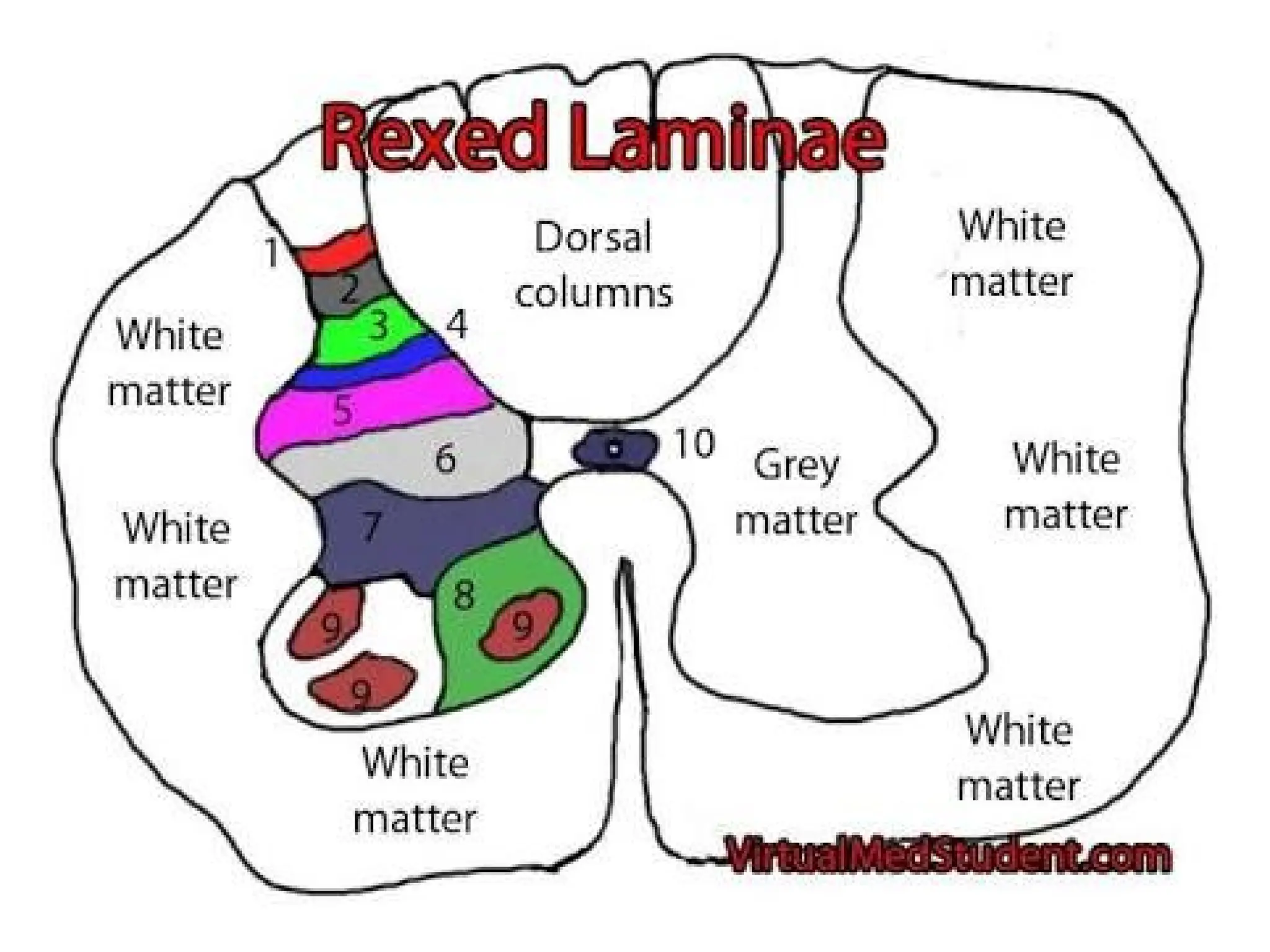
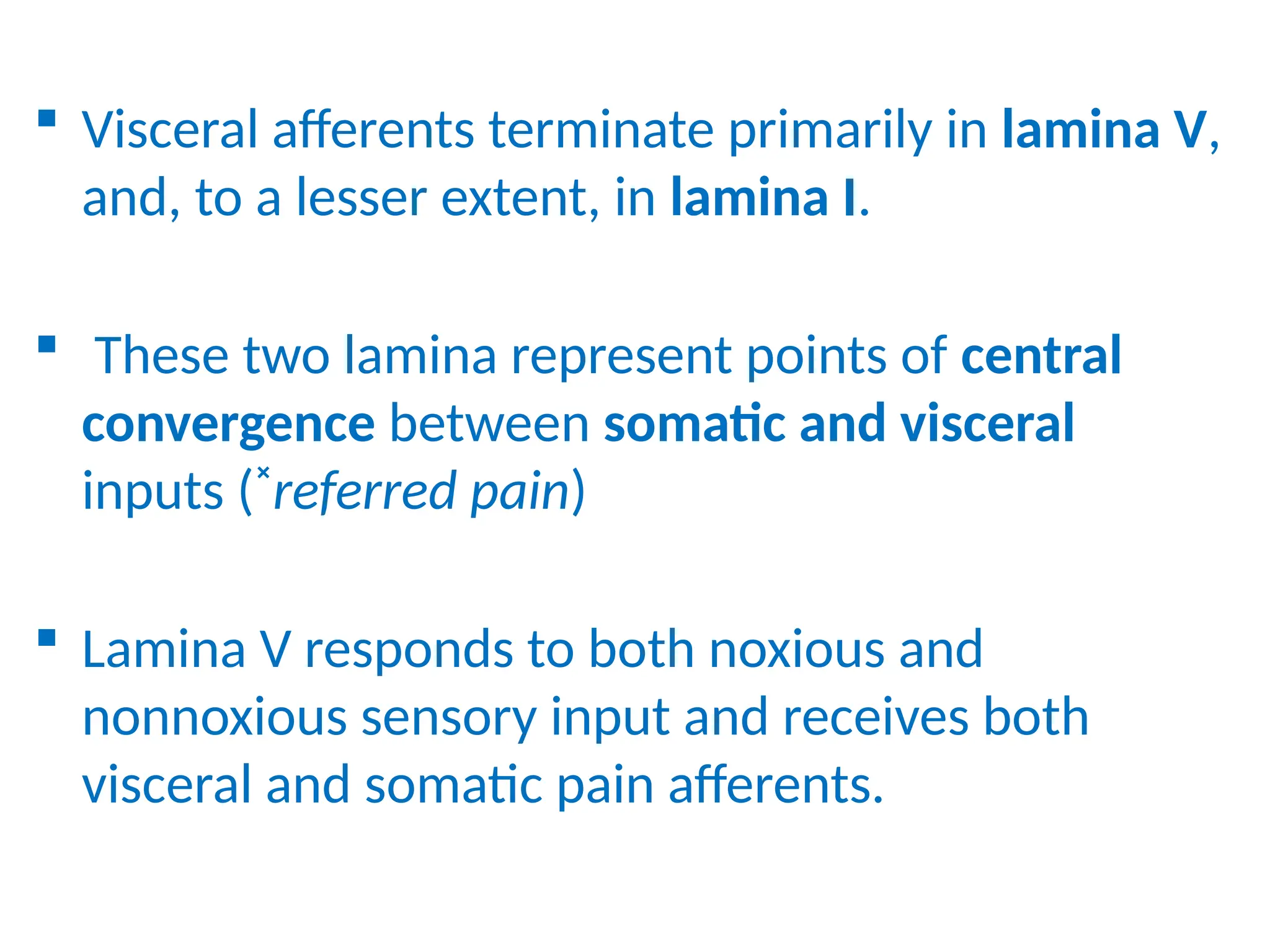
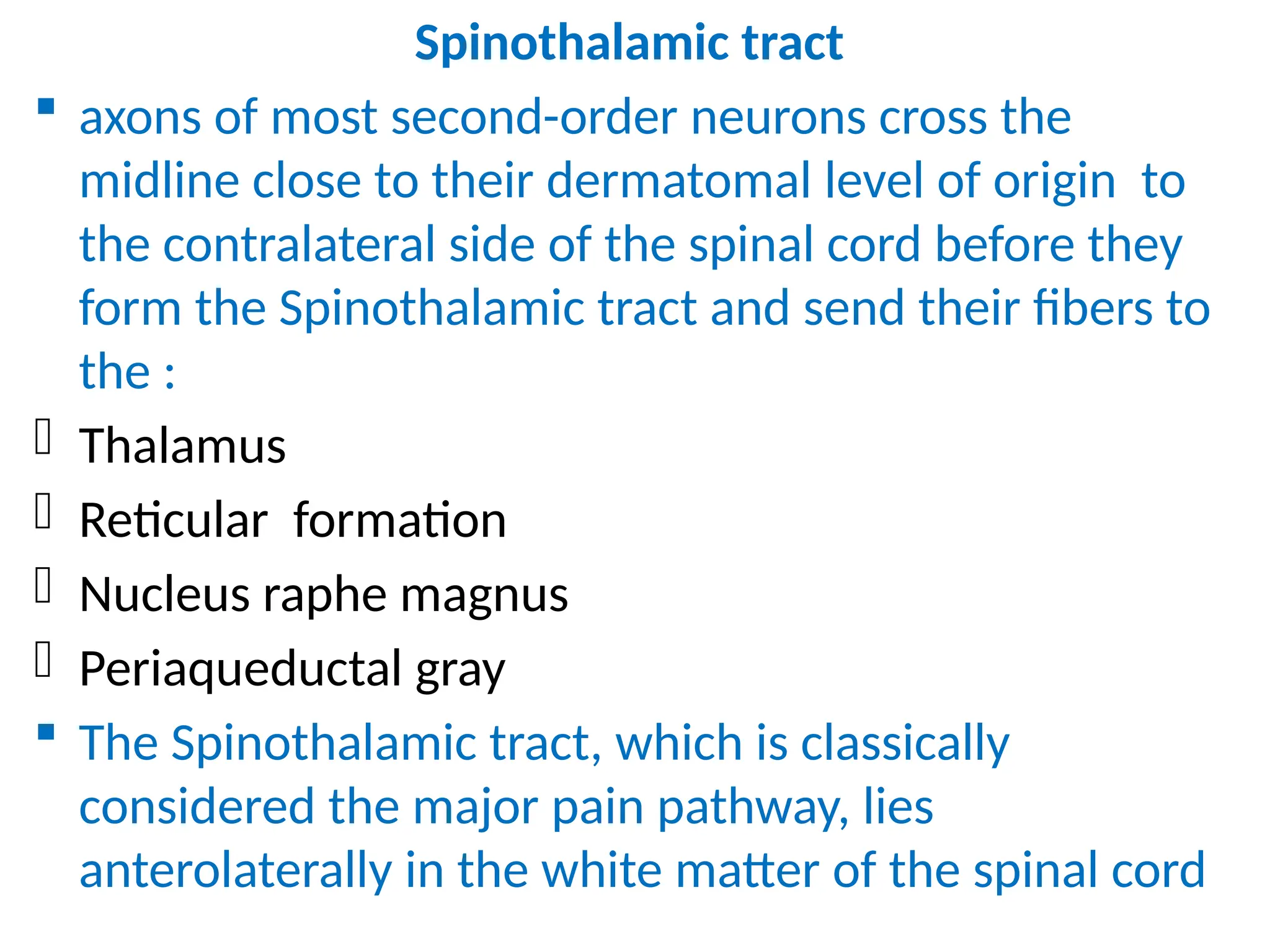
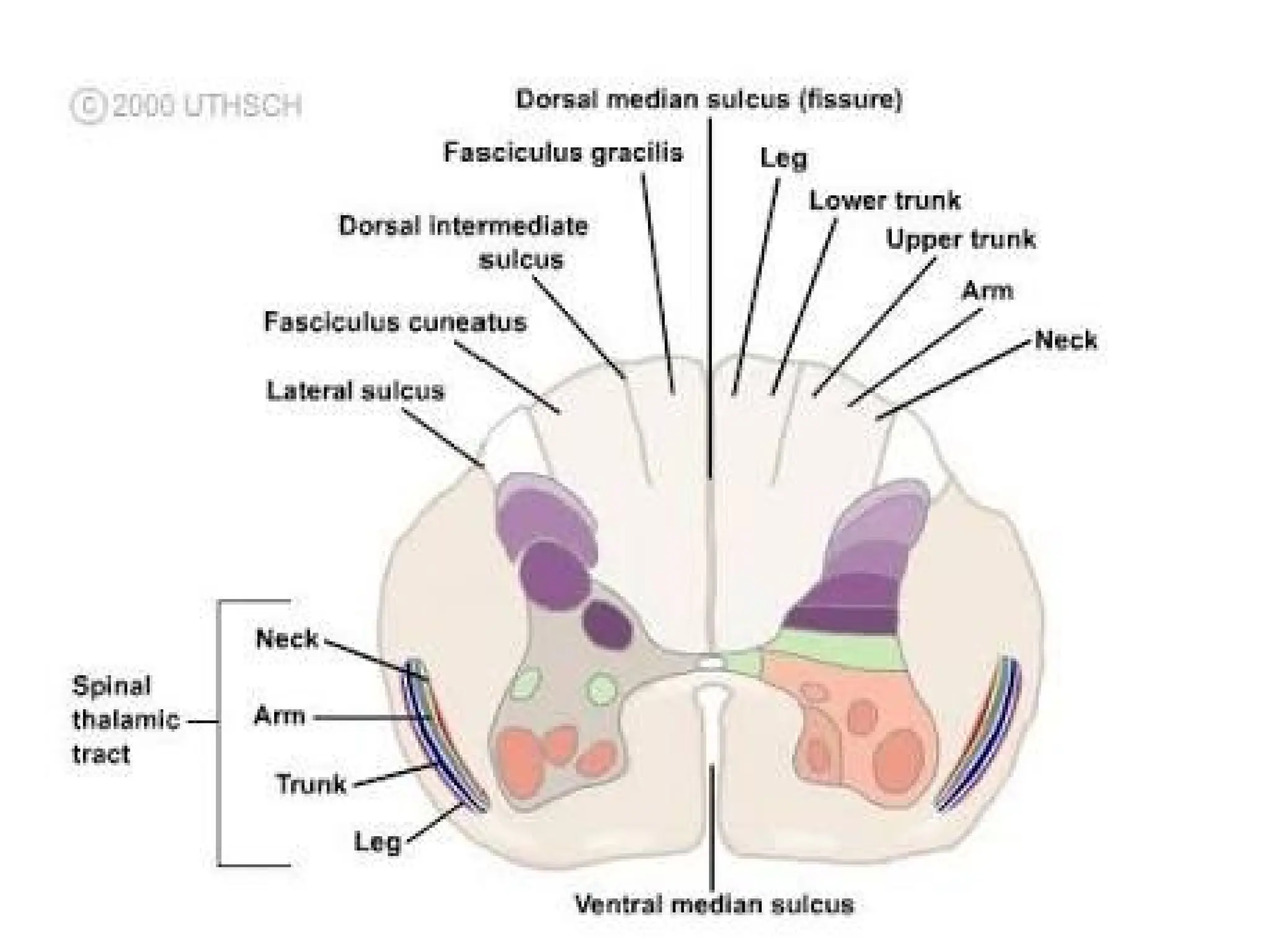
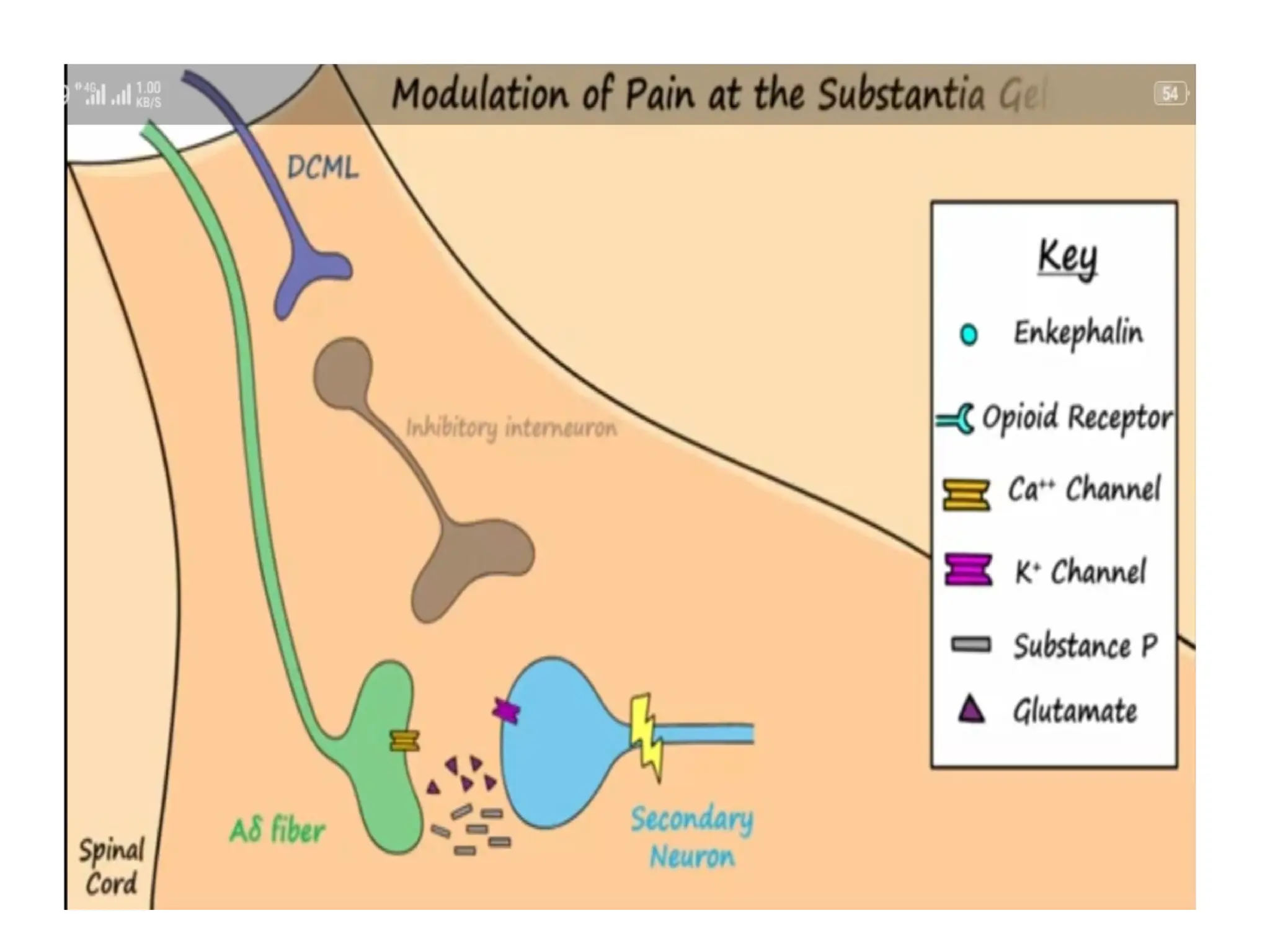
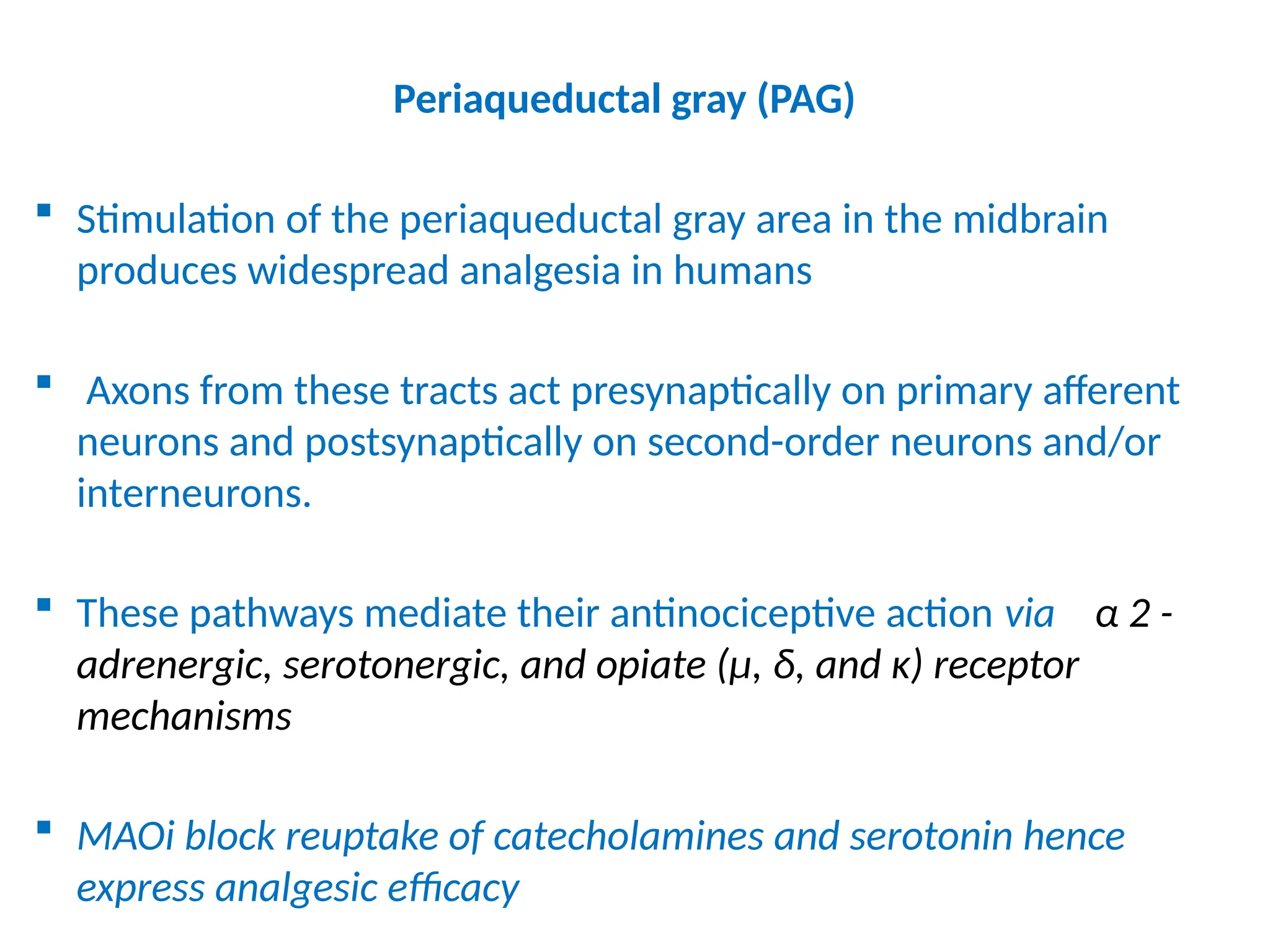
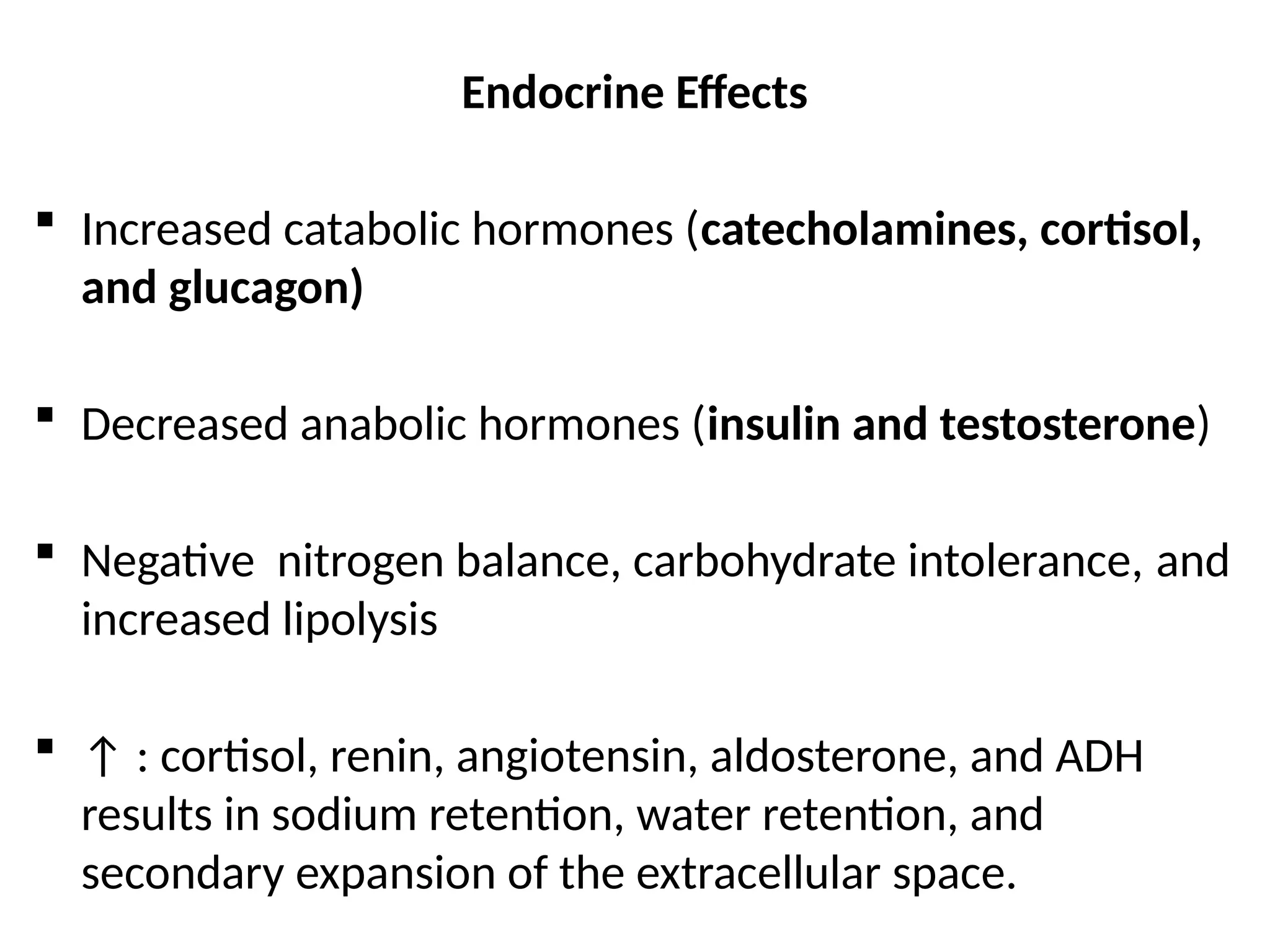
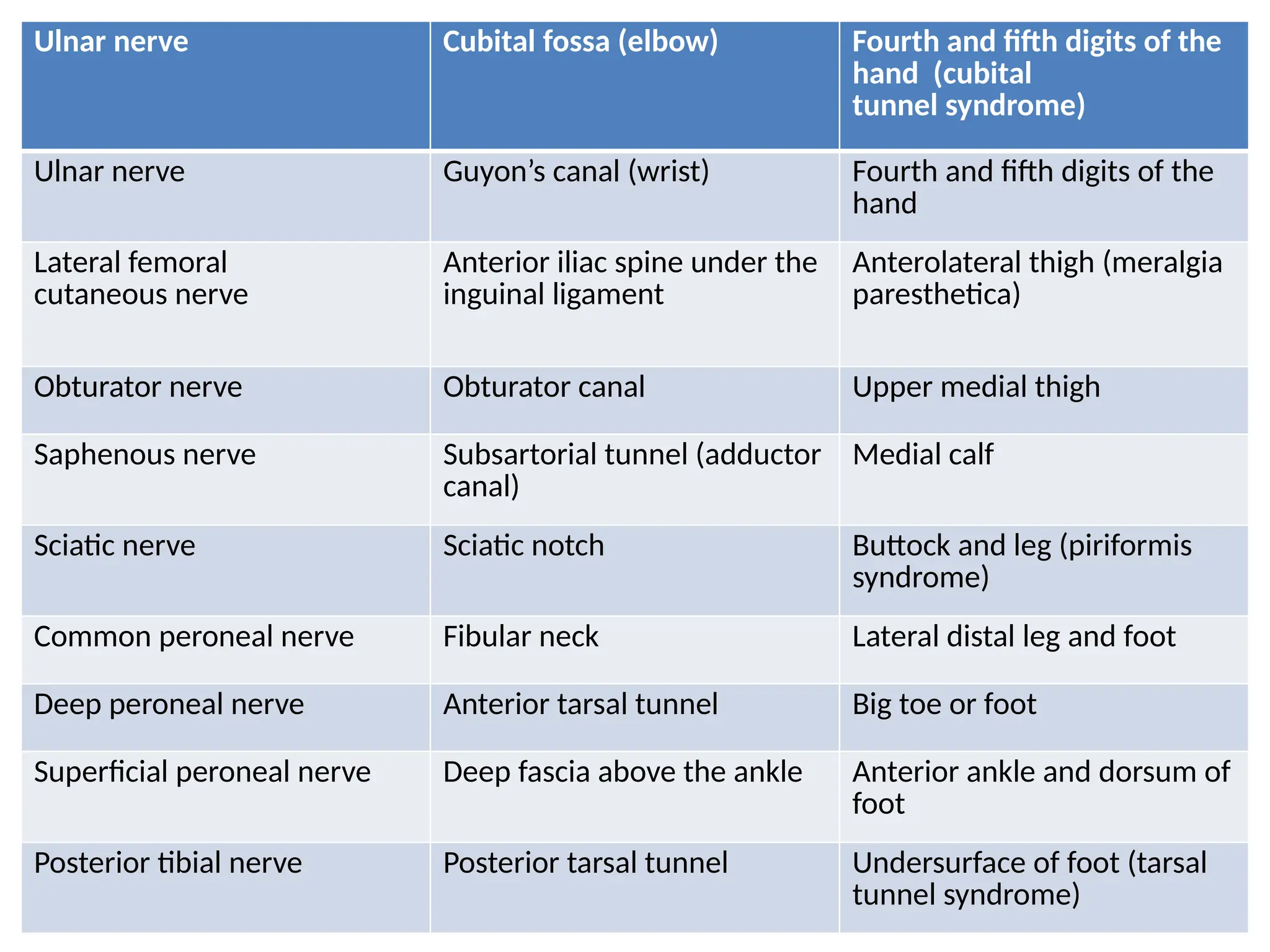
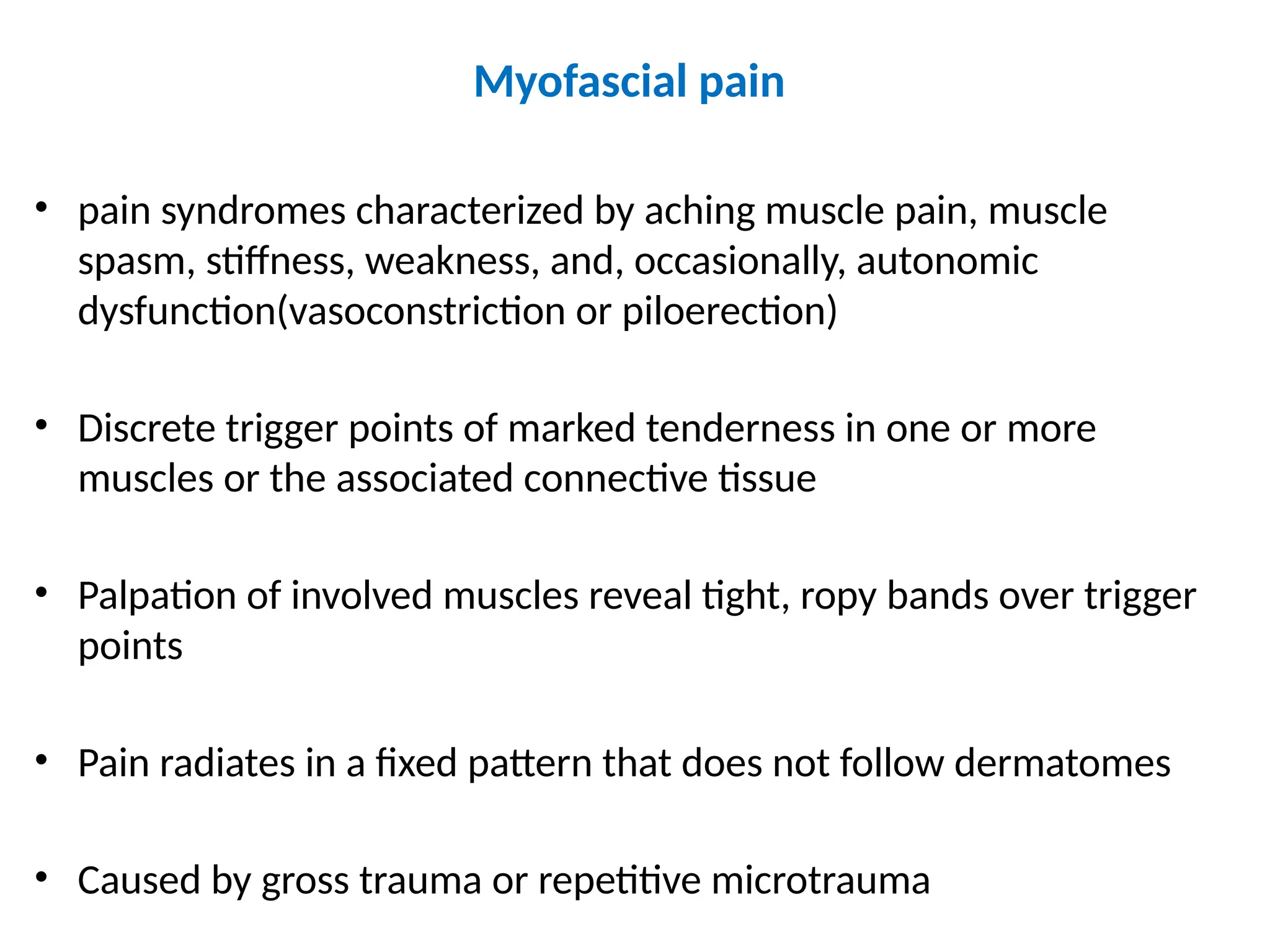
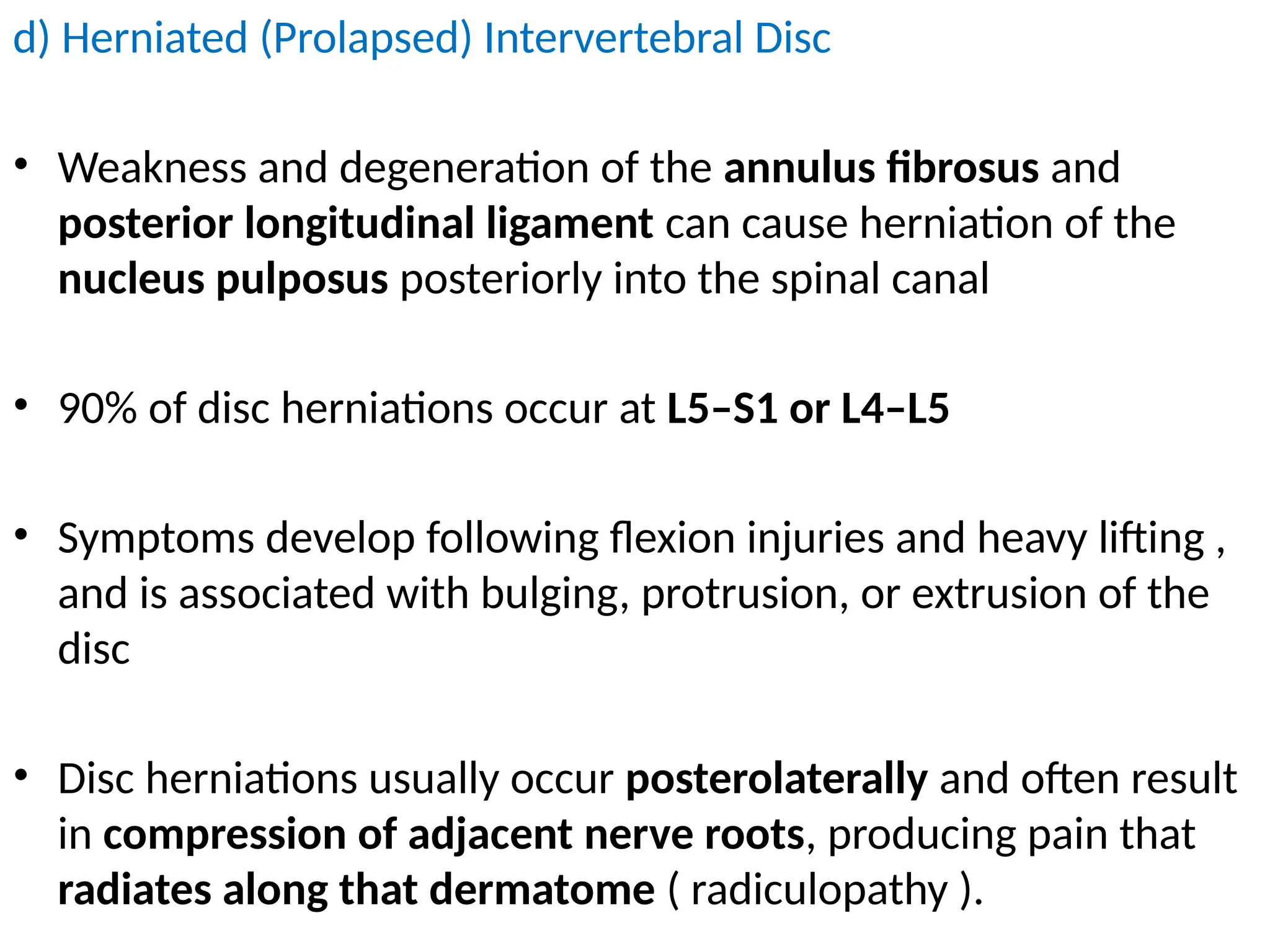
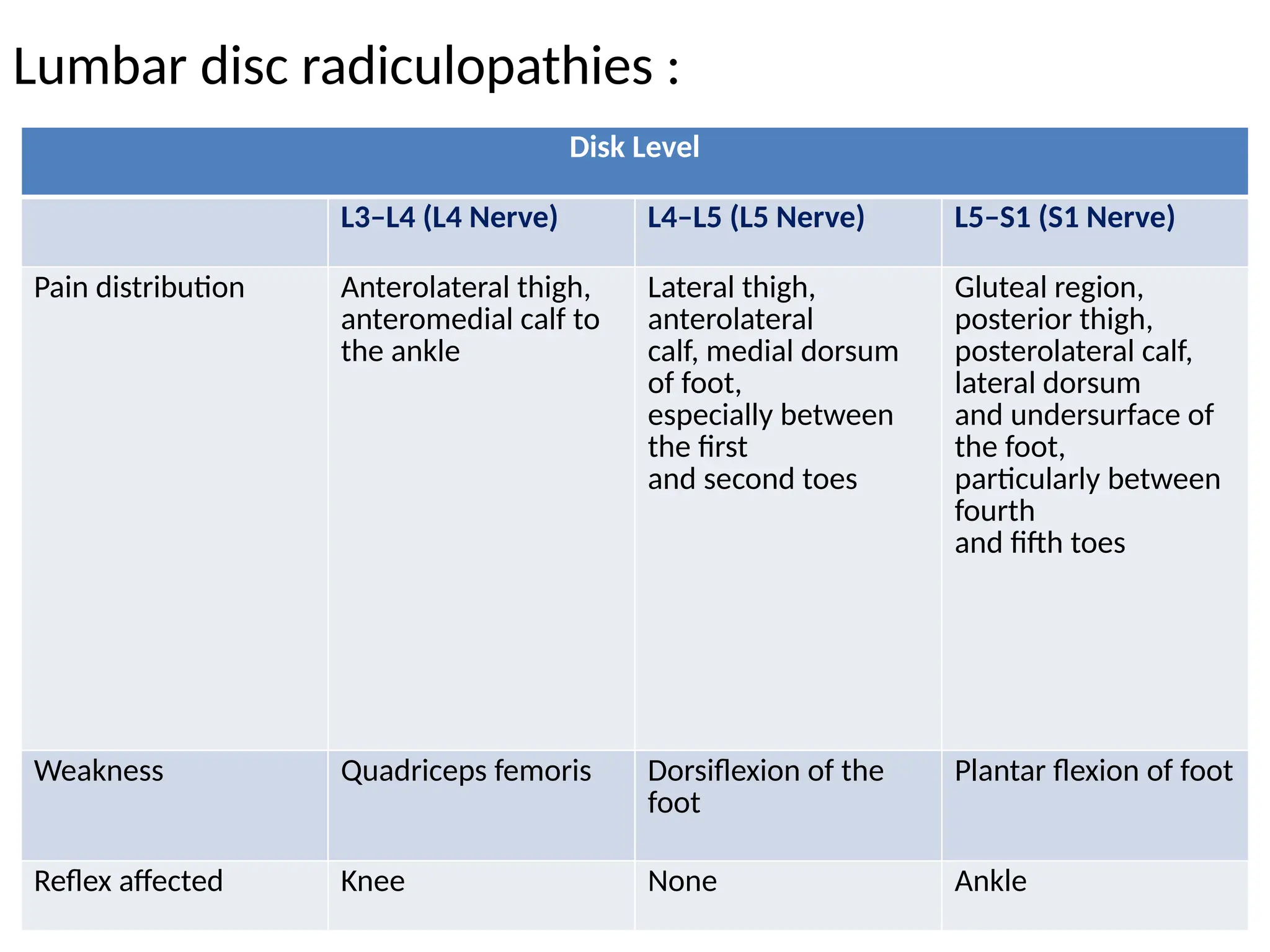
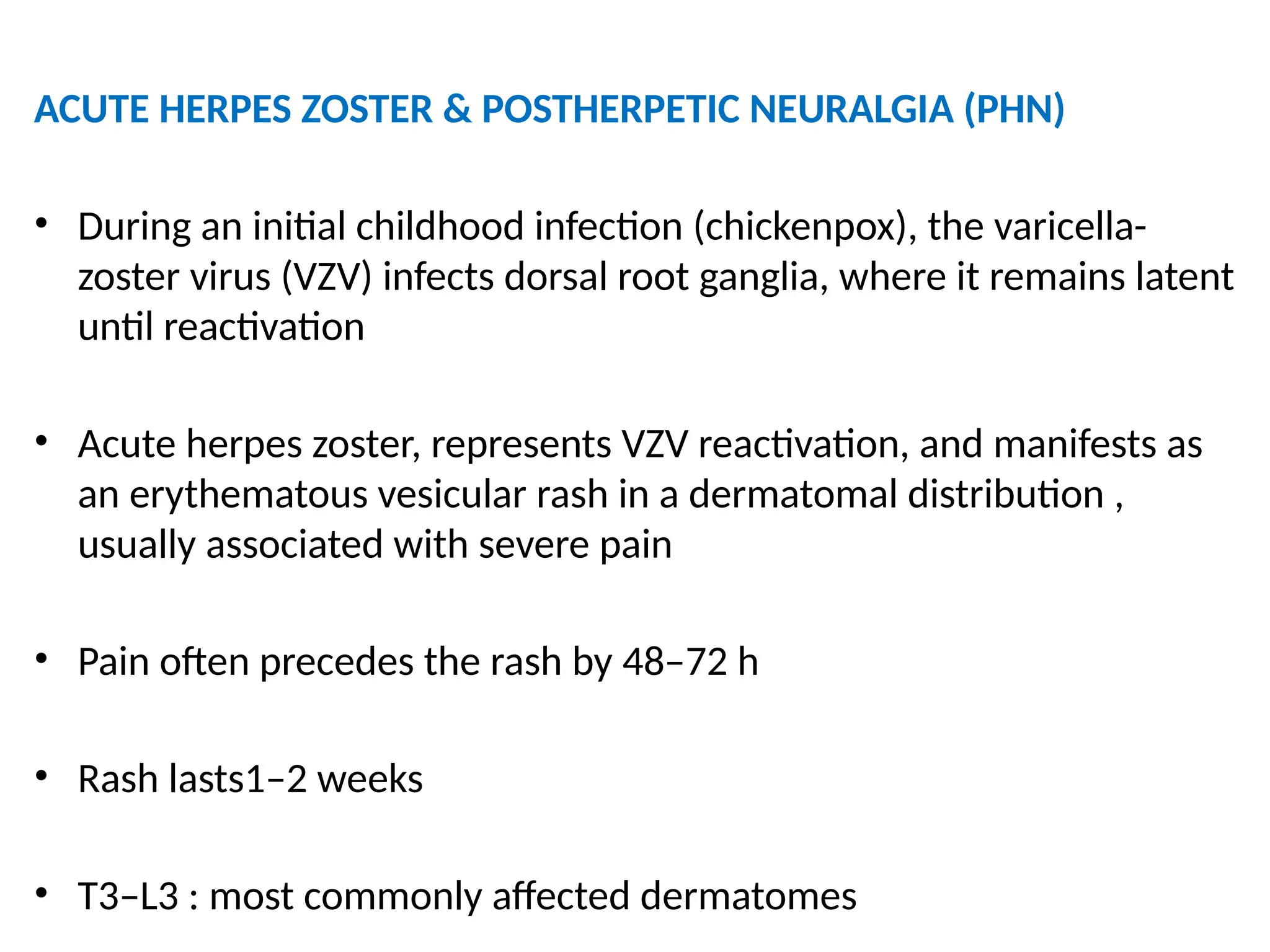
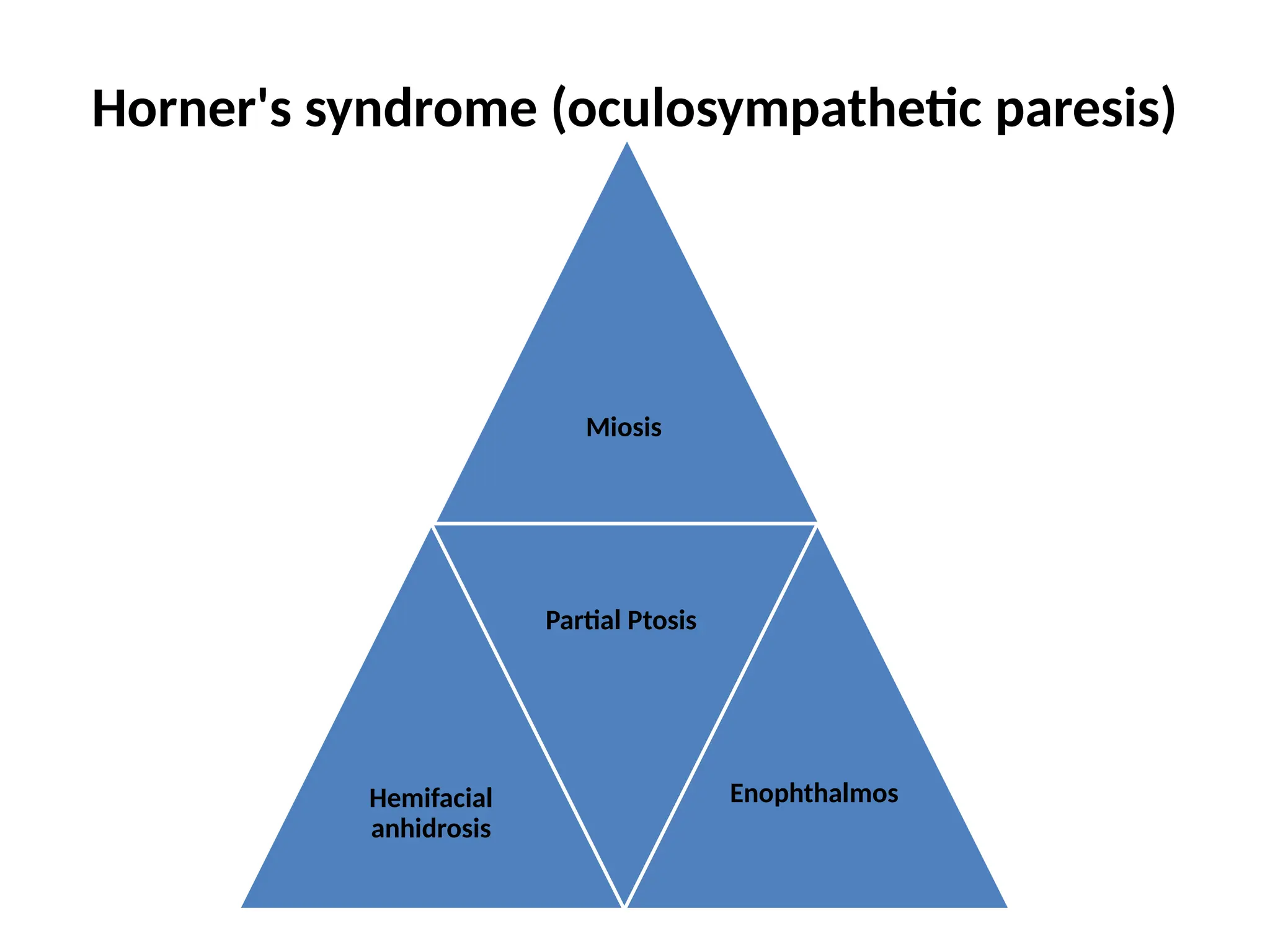
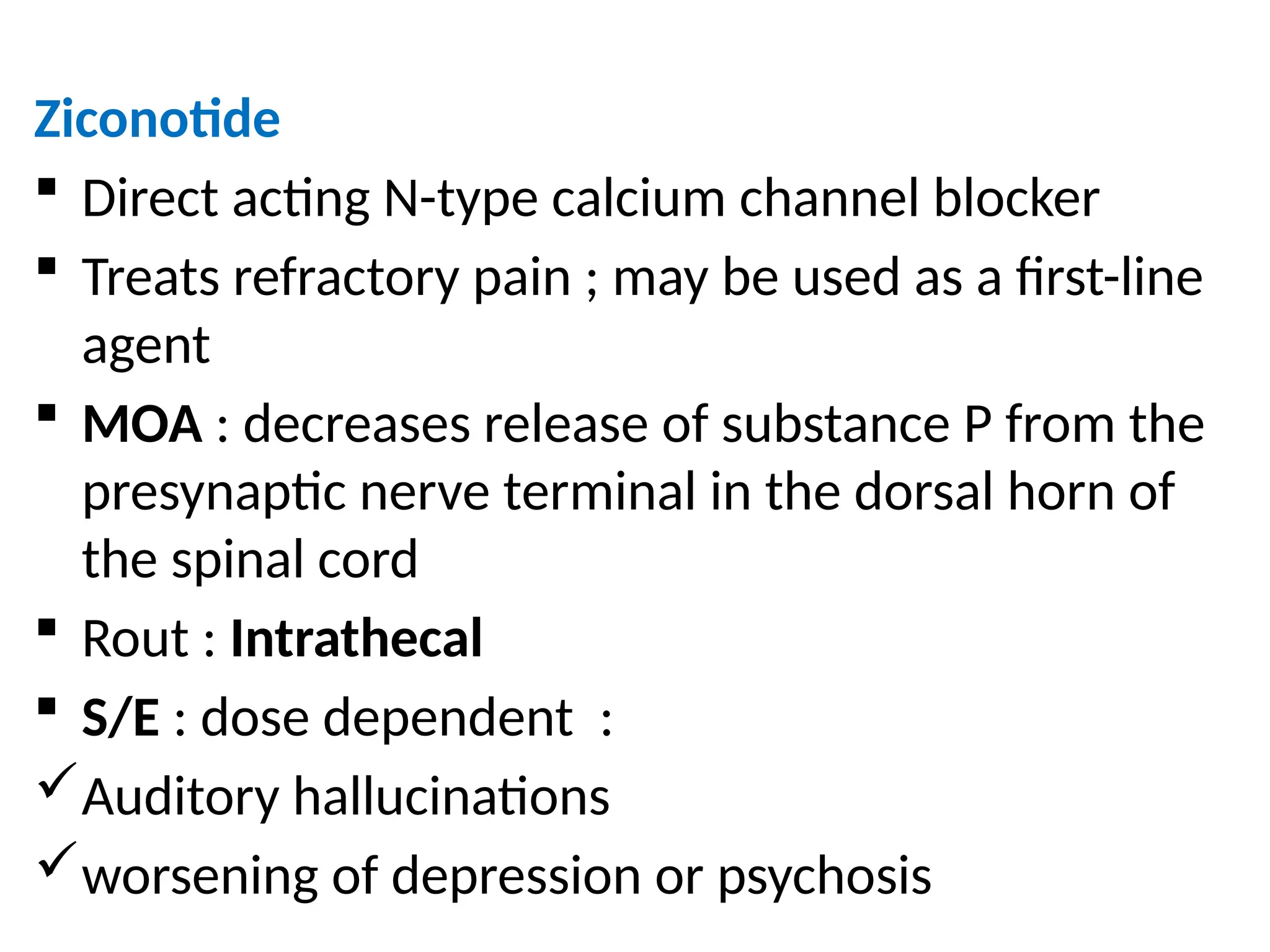
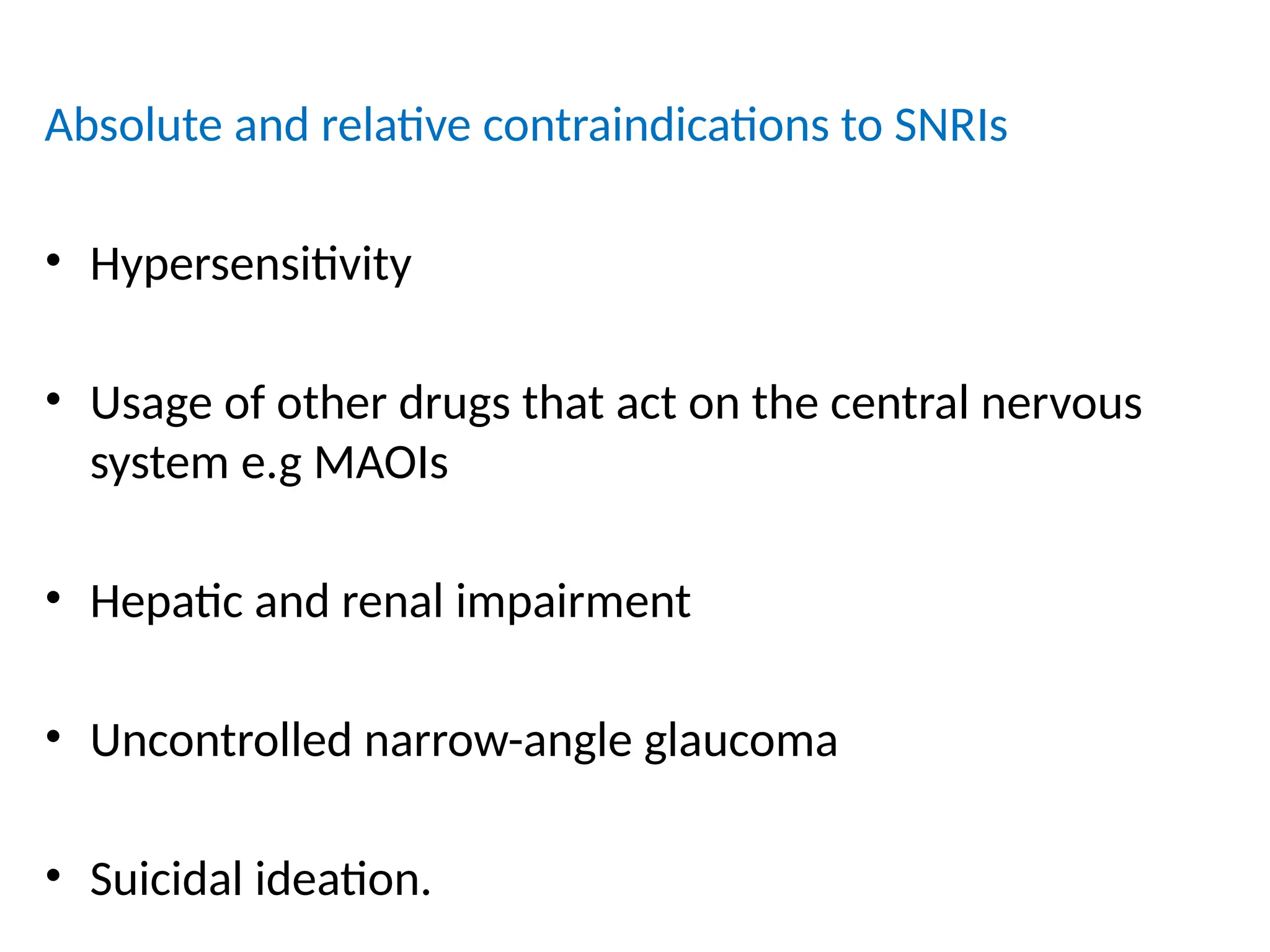
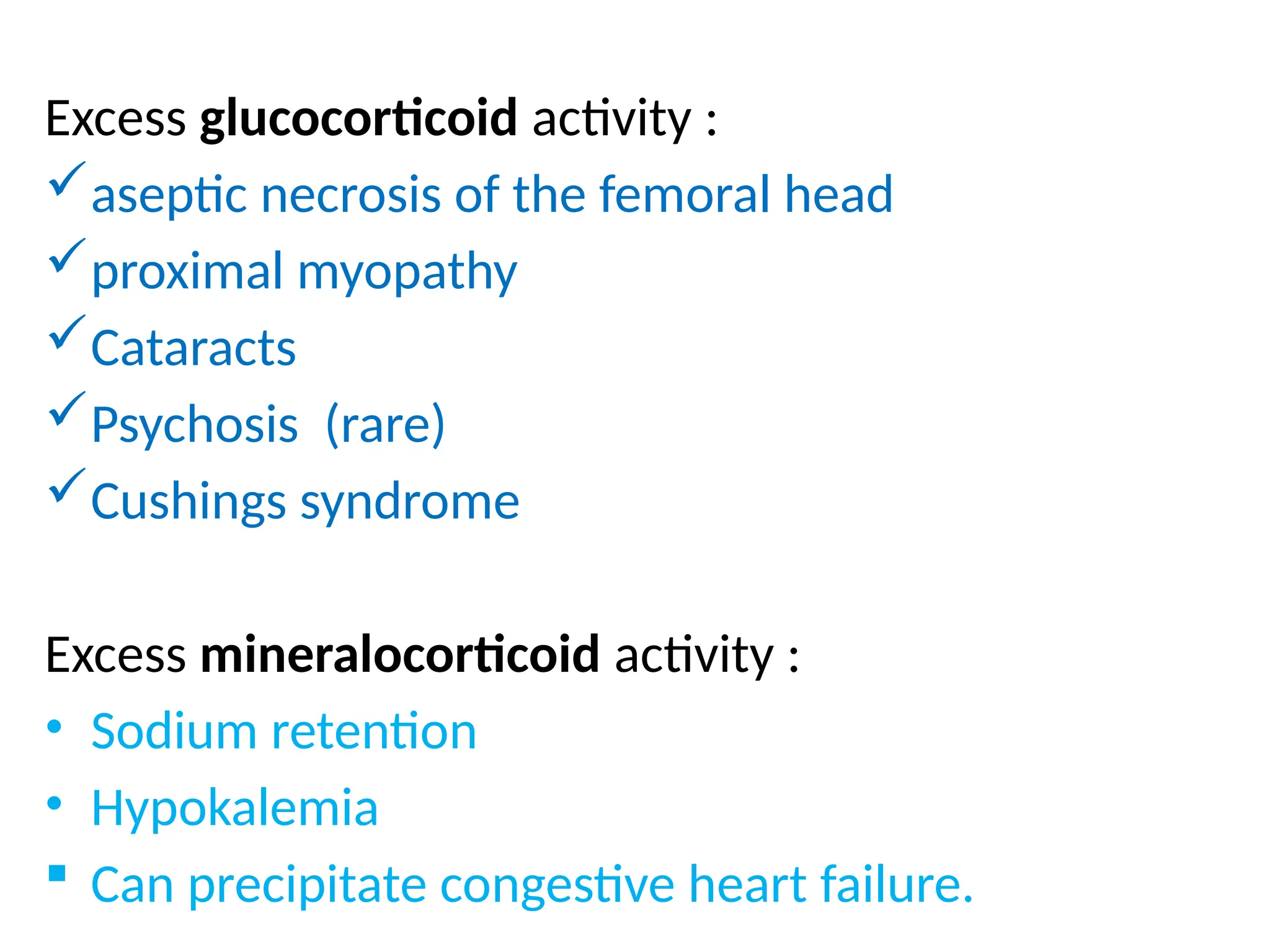
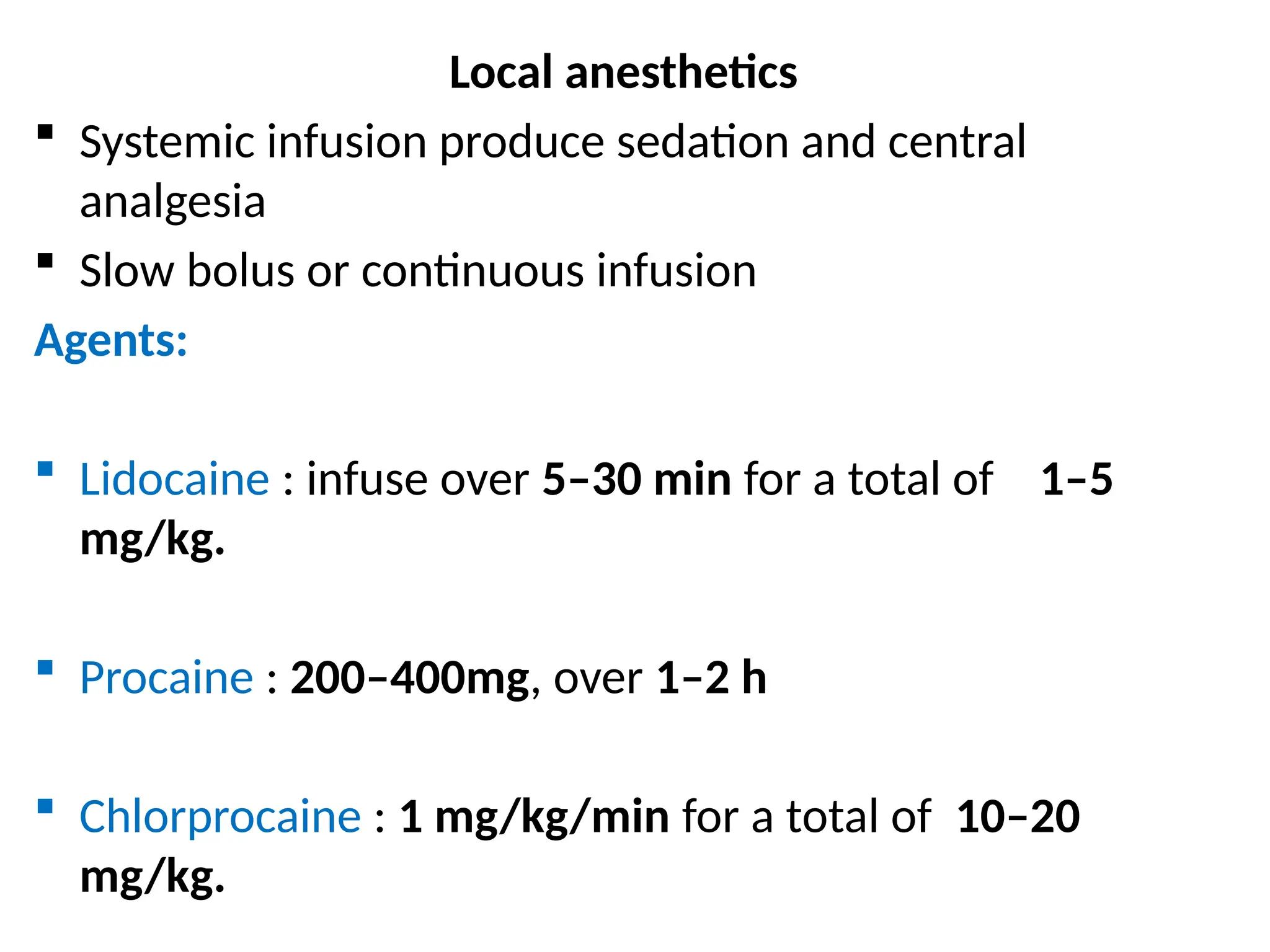
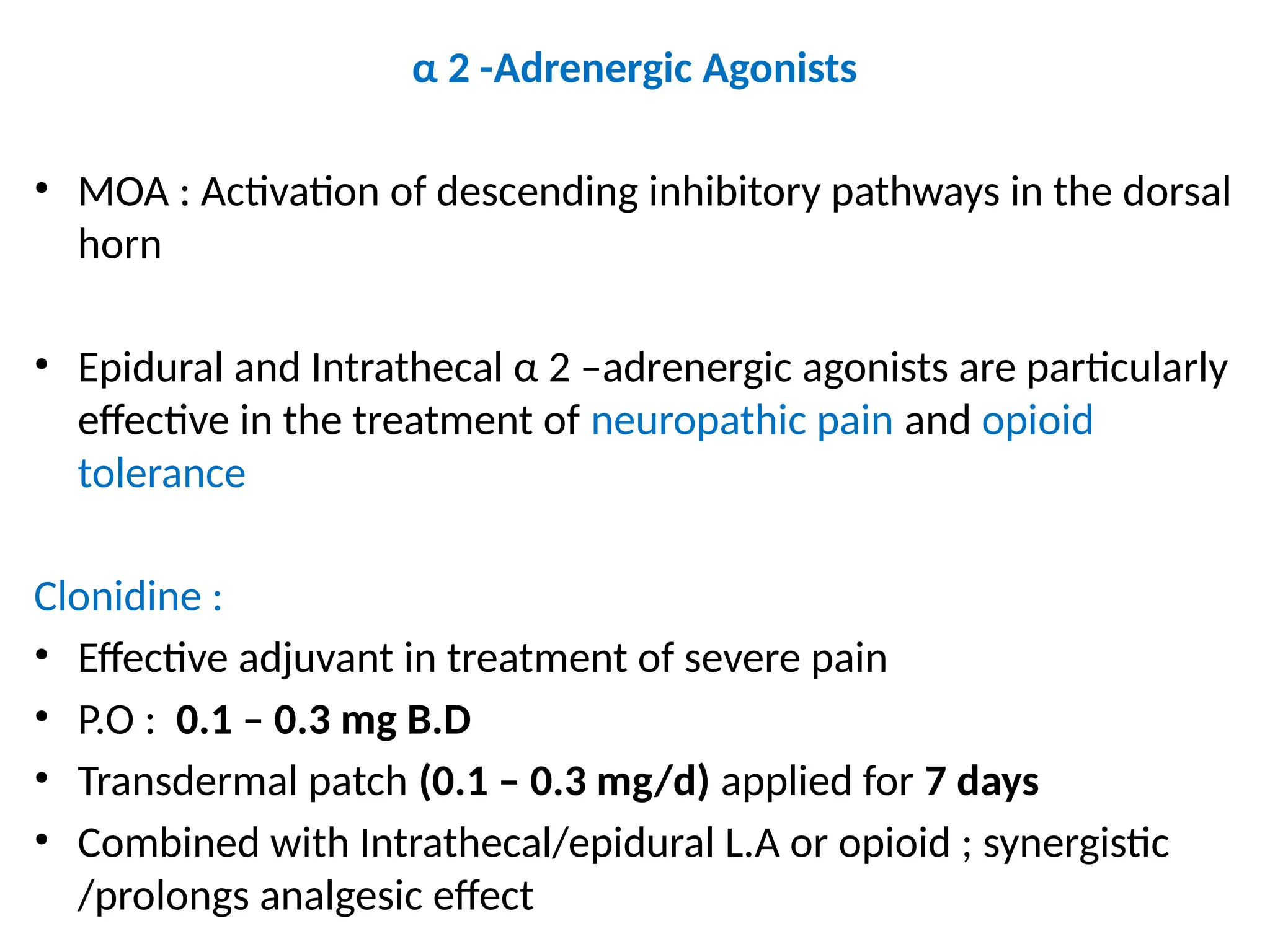
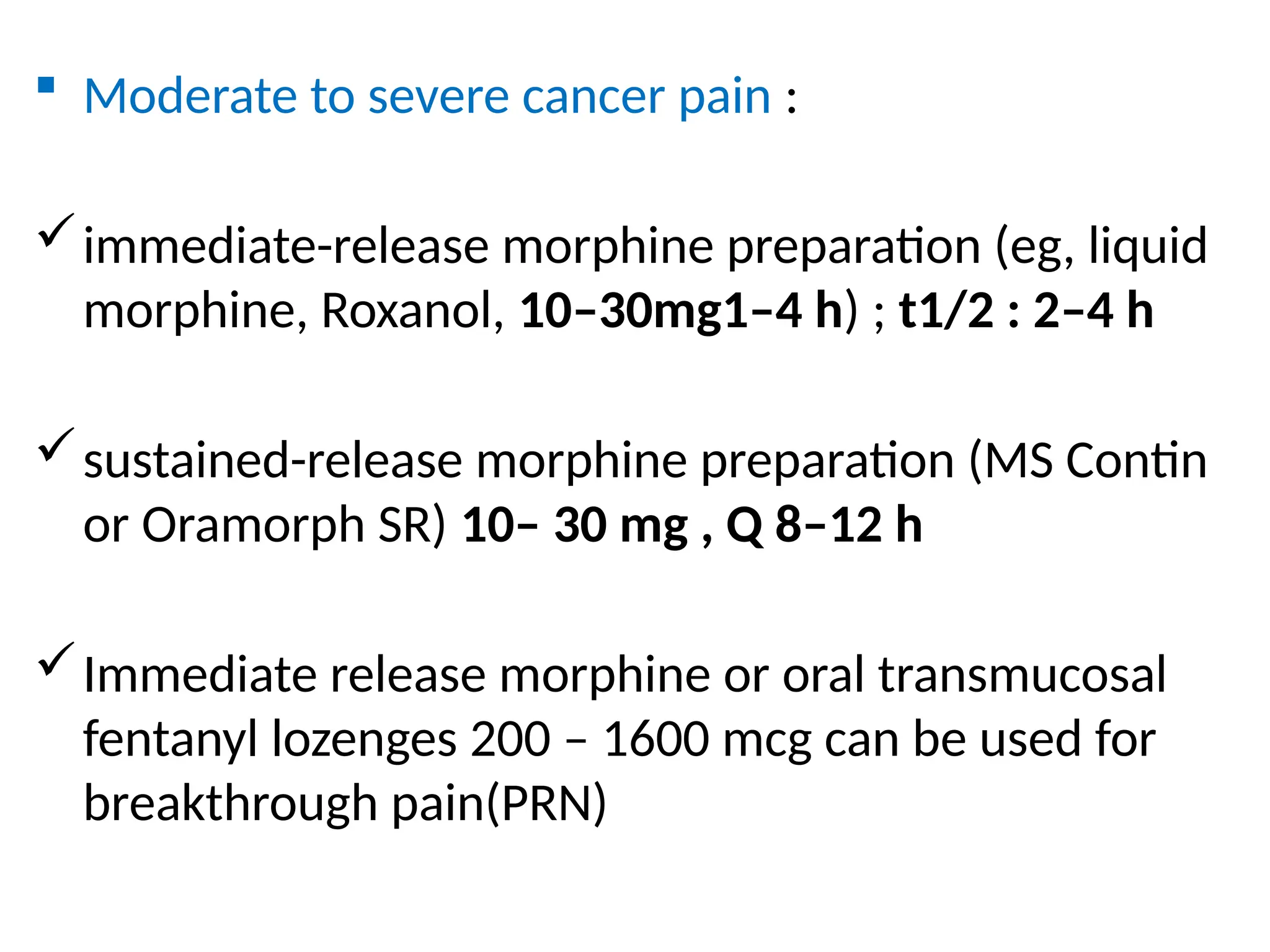
The document provides a detailed overview of pain management, categorizing pain into various types such as nociceptive, neuropathic, acute, and chronic pain. It discusses the physiological processes involved in pain perception, classification, and mechanisms, including the roles of different nerve fibers and pathways. Additionally, it covers concepts like referred pain, various pain syndromes, and the neurobiological underpinnings of pain signaling and sensation.