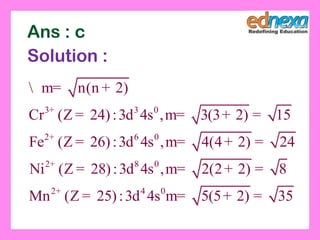
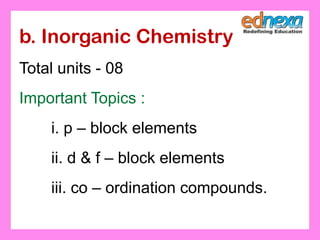
This document discusses the syllabus for the JEE chemistry exam, which is divided into 3 sections: physical chemistry, inorganic chemistry, and organic chemistry. It provides details on the topics covered and number of units in each section. These include chemical thermodynamics, kinetics, and bonding in physical chemistry; p-block and d-block elements and coordination compounds in inorganic chemistry; and organic compounds containing oxygen, halogen, and basic principles of organic chemistry. It also contains example questions and solutions to help understand various chemistry concepts.





![1. The complex used as an
anti-cancer agent is
(a) cis[PtCI2(NH3)2]
(b) cis-K2[PtCI2Br2]
(c) trans-[Co(NH3)3CI3]
(d) Na2CO3
Ans : a](https://image.slidesharecdn.com/chemistrytipsforjeemainmh-cet-140328054053-phpapp01/85/Chemistry-tips-for-JEE-Main-MH-CET-2014-8-320.jpg)
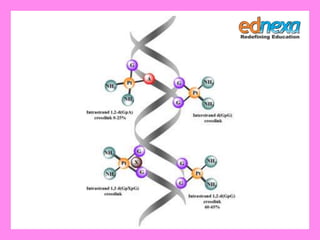
![Solution : Cis-isomer of [Pt (NH3)2 CI2]
is used as an anticancer drugs for
treating several type of malignant
tumours when it is inject into the blood
stream the more reactive CI groups are
lost so, the Pt atom bonds to a N-atom
in guanosine (a part of DNA). This
molecule can bond to two different
guanosine units and by bridging
between them it upsets the normal
reproduction of DNA.](https://image.slidesharecdn.com/chemistrytipsforjeemainmh-cet-140328054053-phpapp01/85/Chemistry-tips-for-JEE-Main-MH-CET-2014-9-320.jpg)