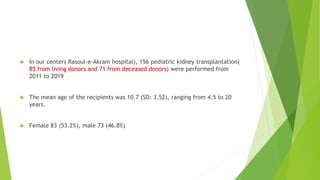
This document discusses challenges with calcineurin inhibitor (CNI) nephrotoxicity in kidney transplantation. It notes that while CNIs like cyclosporine and tacrolimus improved graft survival, they can cause acute and chronic nephrotoxicity. Tacrolimus may have less nephrotoxicity at lower doses without compromising outcomes. The document also outlines risk factors for CNI nephrotoxicity and strategies for prevention, including reduced CNI exposure and renin-angiotensin system inhibitors. It provides details on the author's center's experience with 156 pediatric kidney transplants from 2011 to 2019.