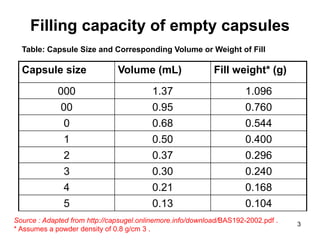
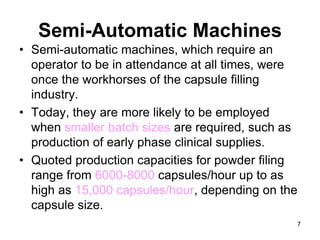
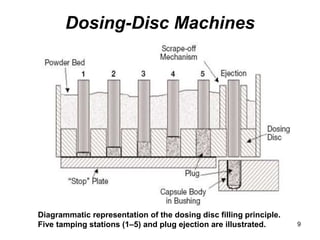
This document discusses different types of capsules used in pharmaceutical manufacturing including empty capsules, soft gelatin capsules, and microencapsulation. It describes the basic components, manufacturing processes, quality control tests, and applications of each type of capsule. The key information provided includes the sizes of empty capsules commonly used, the filling capacities and volumes of different sizes, and the main components and production methods for soft gelatin capsules.