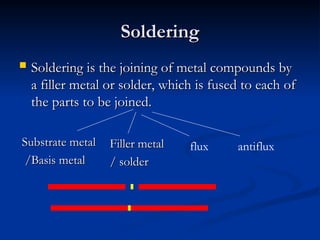
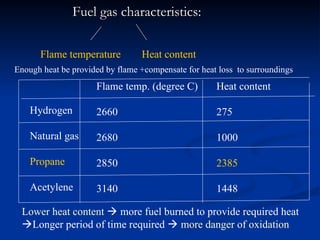
The document covers soldering, a process of joining metals using a filler metal with a lower melting point than the parent metal, emphasizing the roles of various components such as substrate metal, soldering flux, and heat source. It provides definitions and distinctions between soldering, brazing, and welding, as well as details on the types of flux, filler metals, and techniques required for successful soldering. Additionally, it discusses laser welding and the importance of technique and temperature control in the soldering process.