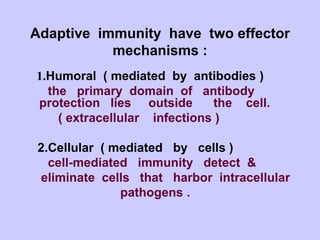
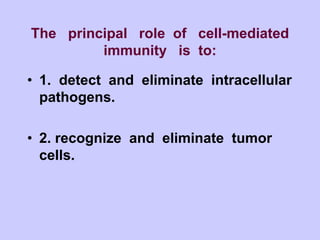
This document summarizes cell-mediated immunity. It discusses that adaptive immunity has two effector mechanisms: humoral immunity mediated by antibodies, and cellular immunity mediated by cells that detect and eliminate intracellular pathogens. Cell-mediated immunity is carried out by cytotoxic T cells, helper T cells, natural killer cells, macrophages, and neutrophils. The activation of naive T cells requires interaction with antigen-presenting cells and co-stimulatory signals. Effector T cells secrete cytokines and express surface molecules that activate macrophages and induce target cell death. T regulatory cells also play an important role in regulating immune responses.