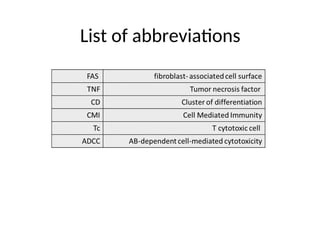
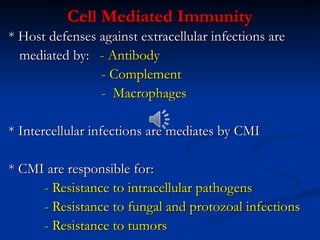
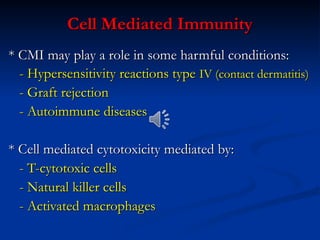
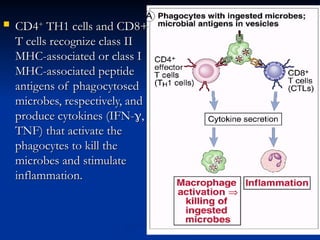
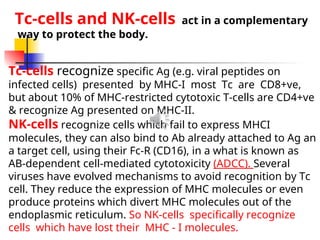
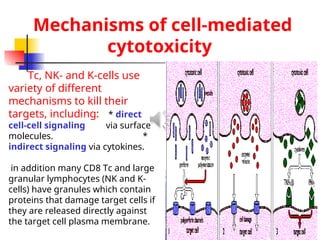
The document discusses cell-mediated immunity (CMI) and its two primary forms: CMI and humoral immunity. CMI is vital for fighting intracellular pathogens and tumors, with T-cells and natural killer cells executing cytotoxic functions, including mechanisms like granule release and signaling via Fas. Additionally, it highlights the role of immune responses in both protective and harmful conditions, such as hypersensitivity and autoimmune diseases.