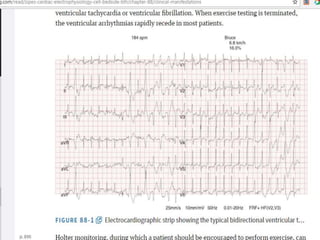
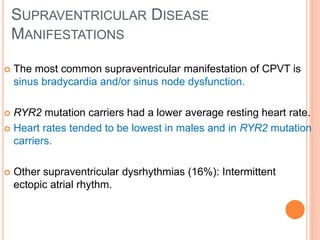
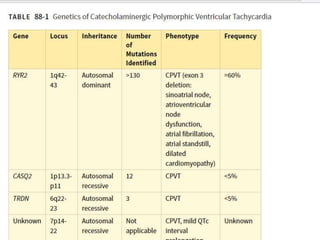
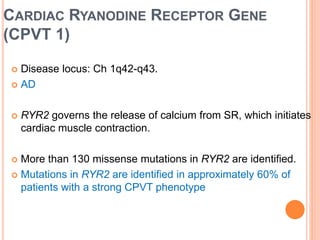
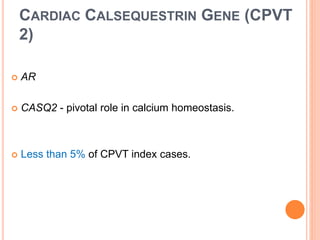
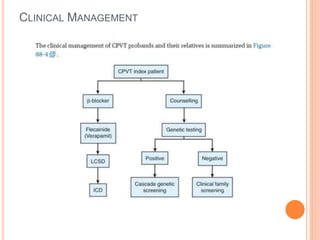
This document discusses catecholaminergic polymorphic ventricular tachycardia (CPVT), a condition characterized by adrenergically mediated polymorphic ventricular arrhythmias without structural heart disease. It has a prevalence of 1 in 10,000 and mortality of up to 50% before age 20 if untreated. The gold standard for diagnosis is exercise stress testing showing exercise-induced bidirectional or polymorphic ventricular tachycardia. Treatment involves lifestyle changes, beta-blockers, flecainide, and an ICD for those with cardiac arrest or recurrent arrhythmias despite medical therapy. Genetic testing identifies mutations in RYR2 or CASQ2 genes in the majority of cases.