1. The student investigated how a small raft made of clear plastic sheet can be propelled by water surface tension. Various liquids like detergent, hand soap, vegetable oil, and salt were dropped on a sponge attached to the back of the raft.
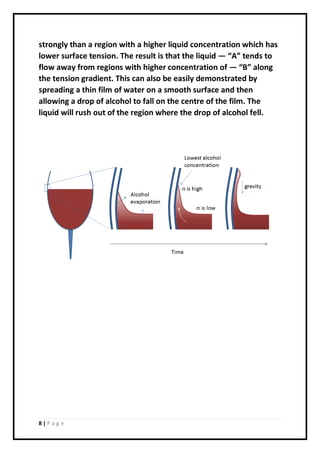
2. When detergent or soap were used, the raft moved across the water's surface due to differences in surface tension caused by the Marangoni effect. Lower surface tension at the sponge caused the water to flow away and propel the raft.
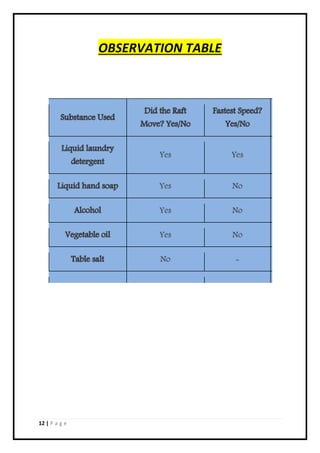
3. Different speeds of motion were observed for each liquid tested, with the ranking being salt>vegetable oil>alcohol>hand soap>detergent based on their respective