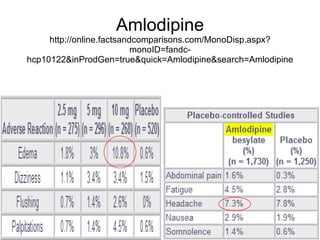
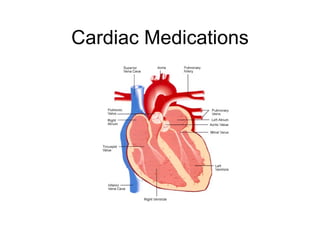
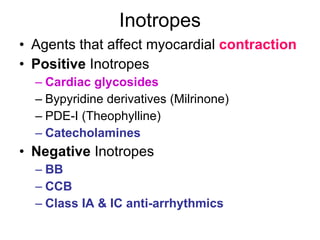
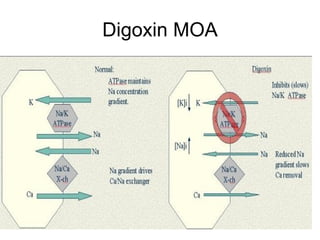
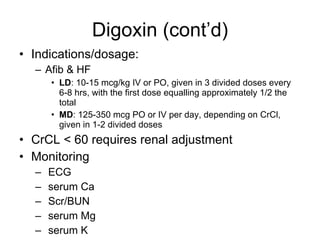
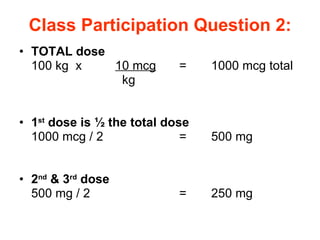
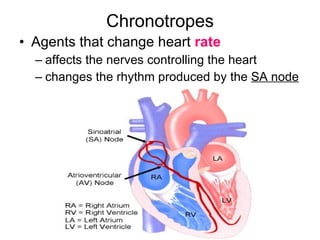
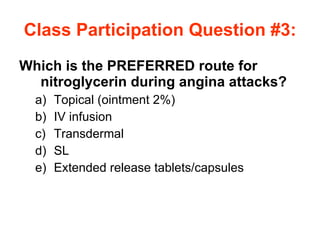
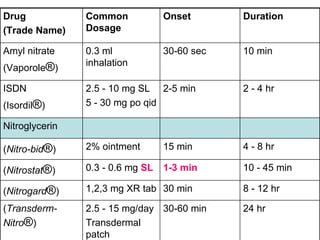
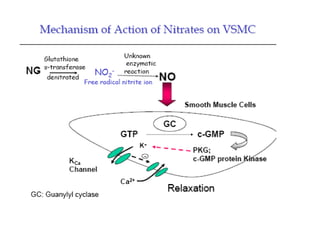
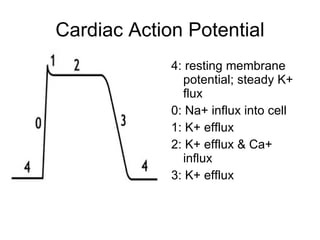
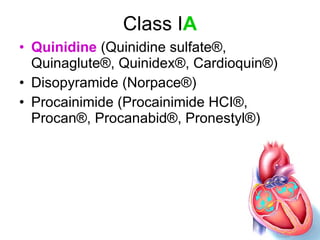
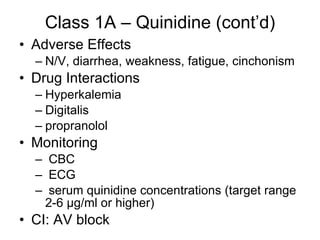
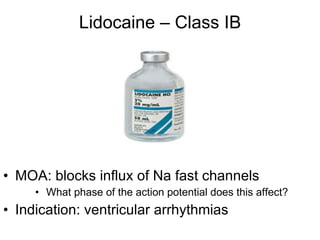
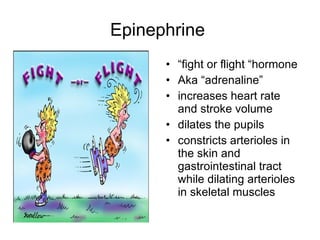
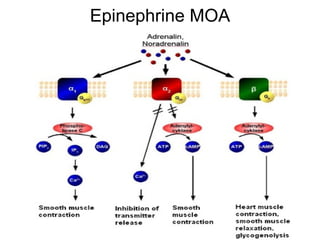
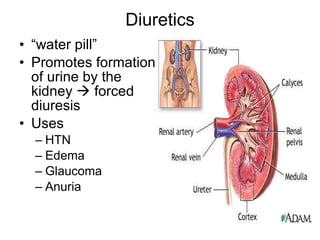
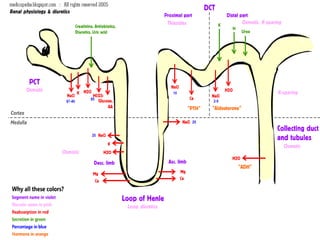
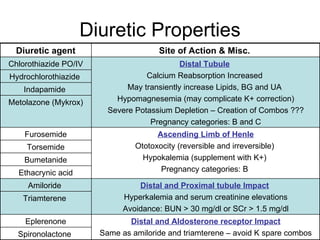
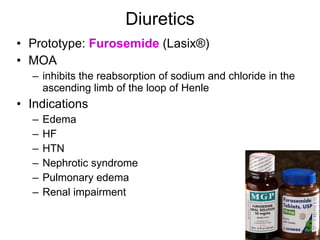
The document provides an overview of various cardiac medications, including their classifications, mechanisms of action, indications, and dosages. It focuses on inotropes like digoxin, chronotropes like atropine, antianginal drugs like nitroglycerin, antidysrhythmics/antiarrhythmics in the four main classes, and discusses specific drugs like quinidine, lidocaine, and flecainide. It includes questions and answers related to calculating digoxin doses and identifying positive inotropes and the preferred route for nitroglycerin during angina attacks.

















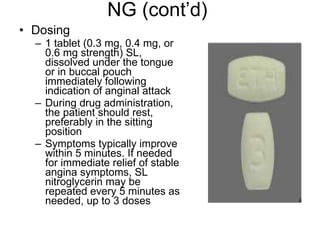






























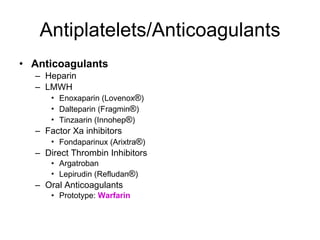

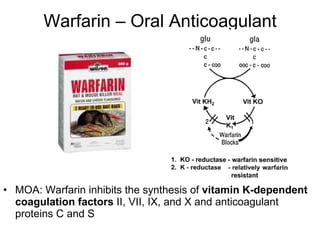
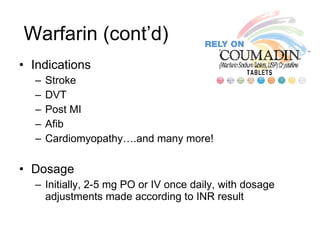
![Warfarin Warnings Bleeding Risk! Warfarin can cause major or fatal bleeding . Bleeding is more likely to occur during the starting period and with a higher dose (resulting in a higher international normalized ratio [INR]). Risk factors for bleeding include high intensity of anticoagulation (INR of more than 4), 65 years of age and older, highly variable INRs, history of GI bleeding, hypertension, cerebrovascular disease, serious heart disease, anemia, malignancy, trauma, renal function impairment, concomitant drugs, and long duration of warfarin therapy. Regular monitoring of INR should be performed on all treated patients . Those at high risk of bleeding may benefit from more frequent INR monitoring, careful dose adjustment to desired INR, and a shorter duration of therapy. Patients should be instructed about prevention measures to minimize risk of bleeding and to report immediately to health care provider signs and symptoms of bleeding Pregnancy Category X](https://image.slidesharecdn.com/cardiacmedications-100913141430-phpapp01/85/Cardiac-medications-91-320.jpg)