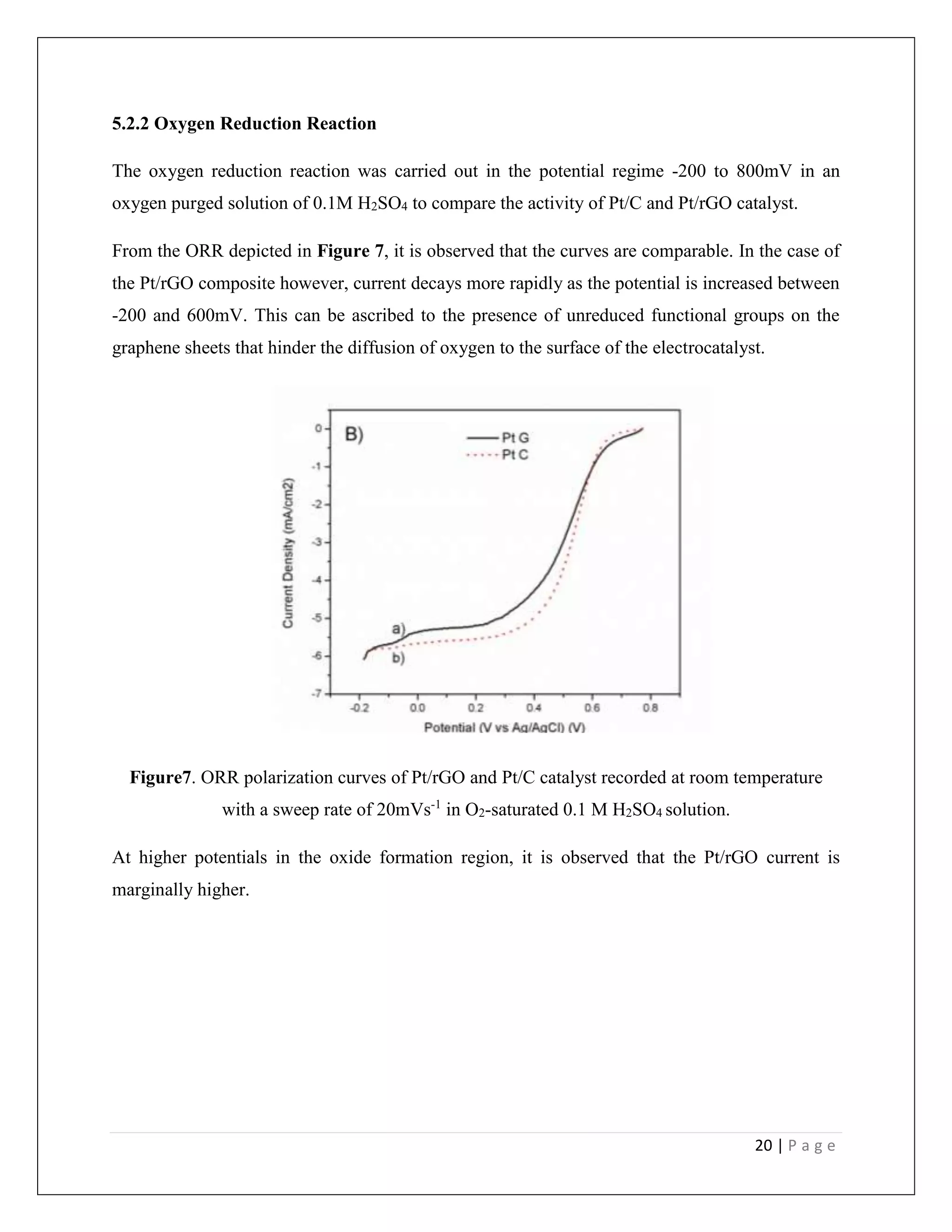
This document is a student project report on platinum-graphene nanocomposites as electrocatalysts in PEM fuel cells. It was submitted by T.V. Sridharan to Professor Manoj Neergat at the Indian Institute of Technology, Bombay under his supervision. The report describes the synthesis of Pt/rGO nanocomposites using a modified polyol method, and their physical and electrochemical characterization. TEM analysis showed the successful deposition of platinum nanoparticles on graphene oxide sheets. Electrochemical experiments found the Pt/rGO composite had a higher effective surface area than Pt/C, but similar activity for the oxygen reduction reaction. Further research is needed to fully realize graphene's potential as





![6 | P a g e
2. Introduction
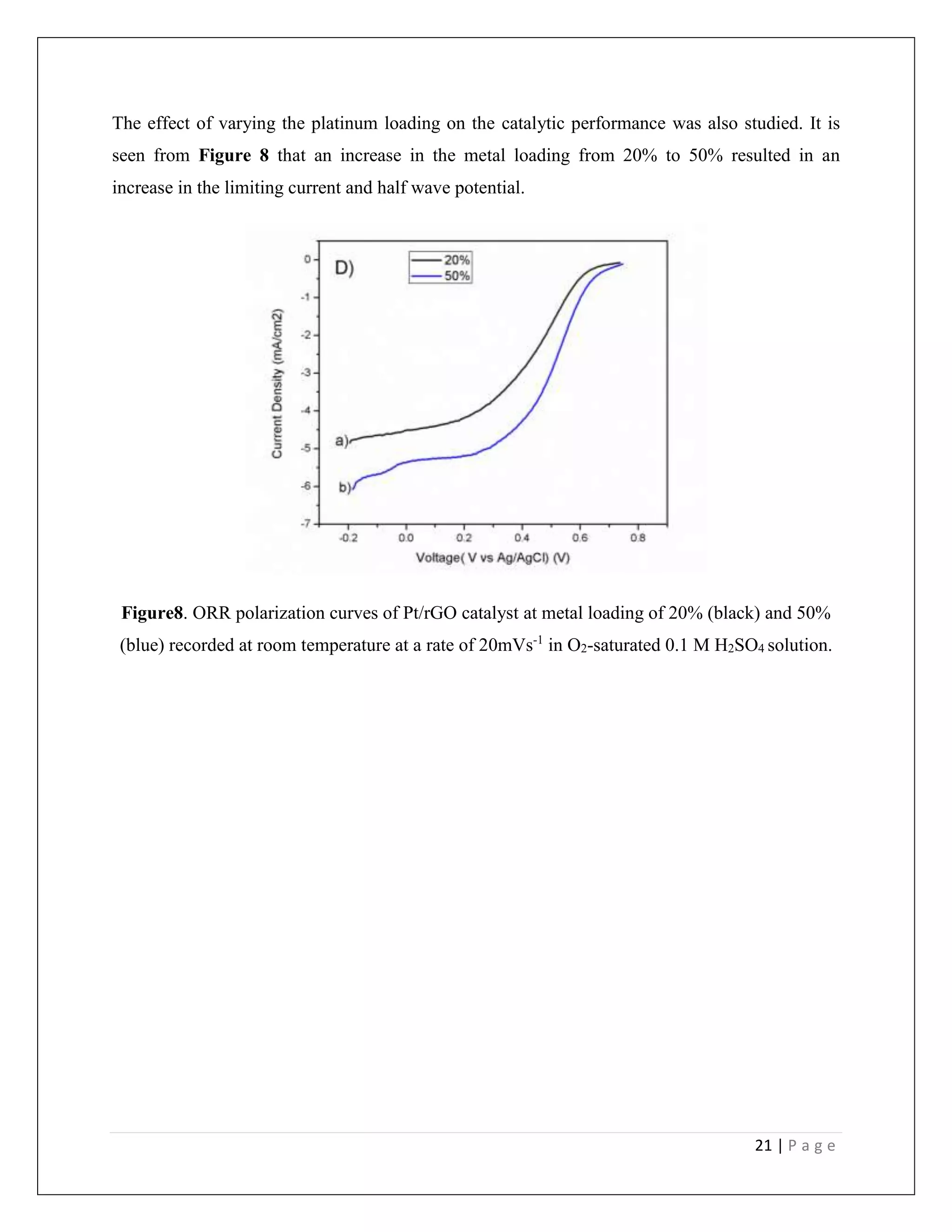
Polymer Electrolyte Membrane fuel cells (PEMFCs) are regarded as promising energy sources
for mobile electronic applications due to their high efficiencies and low operating temperatures.
Their performance and cost are essentially governed by the nature of the electrocatalysts used. It
has long been acknowledged that platinum nanoparticles show superior performance in the
catalysis of the oxygen reduction reaction [1]. However, the commercial success of PEMFCs has
been greatly hindered by high cost of Pt and its ineffective utilization.
One of the prime objectives of PEMFC research is the reduction of precious metal loading on the
electrode without compromising the efficiency of the fuel cell. A great deal of work has focused
on supported metal catalysts which show higher activity and stability compared to unsupported
bulk metal catalysts [2]. A dispersion of the catalyst on support material increases the catalytic
surface area, thereby increasing the utilization efficiency of precious metal. Support materials are
characterized by their surface area, porosity, electrical conductivity, electrochemical stability and
have a strong influence on the performance and durability of catalysts.
Carbon black, because of its high surface area and low cost, has been extensively used as a
support material in PEMFCs [3]. However, carbon blacks are impaired by problems such as, (i)
the presence of organic impurities (ii) entrapment of catalyst nanoparticles in the deep
micropores making them inaccessible to reactants thus leading to reduced catalytic activity [2].
Furthermore, carbon supports and catalytic metals have been reported to degrade under
prevailing conditions of temperature, humidity and potential at the anode. Corrosion of the
support exacerbates the agglomeration and detachment of the catalyst from support materials [4].
Recently, various carbon based nanostructured support materials have come under investigation.
Graphene, a monolayer of graphite composed of carbon atoms in a honeycomb arrangement has
attracted the attention of a multitude of researchers [5]. This two-dimensional (2-D) material has
a theoretical surface area (2630 m2
g-1
) and high conductivity (103–104 S m-1
) that its use as a
catalyst support [5][6].](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-6-2048.jpg)
![7 | P a g e
The conductive support facilitates efficient collection and transfer of electrons to the electrode
surface. The large specific surface area coupled with its excellent thermal, electronic and
mechanical properties, make graphene a potential alternate substrate for the deposition of
inorganic nanoparticles to produce highly dispersed composites.
In this study, the effective surface area and activity of Pt/C was compared with Pt/G composite
of the same loading of platinum. Further, the catalytic performance of Pt/G nanocomposites with
different weight compositions of platinum and graphene was studied by means of their cyclic
voltammograms and oxygen reduction reaction (ORR) polarization curves. Pt/rGO catalyst was
prepared by simultaneous reduction of H2PtCl6.6H2O and graphene oxide using a modified
polyol method [7]. TEM and XRD were employed to characterize morphology and surface
composition of the samples. The role of graphene as an effective catalyst support was
investigated.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-7-2048.jpg)
![8 | P a g e
3. Literature Review
The unique structural, physical and chemical properties of graphene draw attention to possibility
of the preparation of novel composite materials with superior catalytic properties. Although
single layer graphene catalytic supports have not been reported, promising results from few
layered graphene stacks are encouraging increased research efforts in this direction. Thus it is
important to discuss the properties of graphene which are of relevance to its application as a
catalyst support and its influence on the activity oxygen reduction reaction in PEMFCs.
Graphene has a hexagonal arrangement of carbon atoms in a 2 D plane forming a honeycomb
lattice (Figure 1). The planar sheet structure provides a very high surface area for the attaching
catalyst nanoparticles. The large surface area is an advantage lost when graphene sheets
irreversibly agglomerate. The extraordinary properties of graphene are associated with individual
layers.
Figure 1. Graphene monolayer with a honeycomb structure [20]
The carbon atoms in graphene are said to be sp2 hybridized. The bonds provide a strong
hexagonal backbone, and the out-of plane ∏ bonds are responsible for interaction between
different graphene layers. The lone pairs of ∏ electrons are delocalized and facilitate conduction
of charge through the plane normal to the c-axis of graphite (electron conduction along c-axis is
much lower).
Graphene is considered a zero-band gap semiconductor since the valence and conduction bands
overlap. The electronic properties of graphene vary with the thickness, or the number of stacked
layers. At one atom thickness, graphene is transparent suggesting its application in photocatalytic
reactions.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-8-2048.jpg)
![9 | P a g e
Among the several exciting properties of graphene, the one that has attracted considerable
attention is its adsorption. Transition metals, and specifically platinum and palladium have been
shown to have remarkable catalytic properties. The adsorption mechanism and interaction
between metal atoms and the graphene support has become vital to fabricate graphene-based
composite catalyst materials. Several research groups have reported the performance of
Pt/Graphene composite as effective electrocatalysts.
Soin et al. synthesized Pt/Graphene nanoflakes electrode for the Methanol Oxidation Reaction.
Microwave plasma assisted chemical vapour deposition technique was used to grow the
vertically aligned graphene nanoflakes. Raman spectroscopy confirmed the characteristics of
highly crystallized few layered graphene. Pt nanoparticles were sputtered onto the graphene
nanoflakes. Cyclic Votammetry curves demonstrated fast electron transfer (ET) kinetics for the
Pt/Graphene electrodes. The rapid electron transfer kinetics was attributed to the highly
graphitized edge structure of the nanoflakes [8].
Ali Grinou et a.l used an aniline stabilized Pt/rGO composite for electrochemical studies. The
nanocomposite was reduced by ethylene glycol solution and aniline stabilized the Pt
nanoparticles, without altering the reduced graphene oxide structure. A marked enhancement of
the electrical conductivity of the composite prepared using the aniline stabilizer was reported and
was attributed to the morphological structure, small particle size, uniform dispersion in large
quantities of Pt NPs and good interfacial interaction between the Pt NPs and rGO hybrid. [9]
Jafri et a.l synthesized Pt nanoparticles supported on nitrogen doped graphene by thermal
exfoliation of graphite oxide. This was performed by dispersing the Pt nanoparticles on the
support using the sodium borohydride reduction process. Electrochemical characterization using
Pt/N-G and Pt/G as the ORR catalyst showed a maximum power density of 440mWcm−2
and
390mWcm−2
, respectively. The improved performance of Pt/N-G was attributed to the
incorporation of nitrogen in the C-backbone leading to increase in the conductivity of
neighbouring C atoms [10].](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-9-2048.jpg)
![10 | P a g e
The fast electron transport mechanism ascribed to the presence of graphene facilitates and speeds
up the Oxygen Reduction Reaction in fuel cells. Min Ho Seo et al explored the use of graphene
supported electrocatalysts for the oxygen reduction reaction (ORR) in alkaline medium. Both Pd
and Pt nanoparticles with a mean diameter of 1.8 nm were dispersed on graphene sheets (GNSs)
through chemical synthesis at a metal loading of 60 wt%. The ORR activity of these catalysts
was investigated in a 0.1 M NaOH solution and was reported to show significantly high activity
for ORR [11].
Several synthesis procedures have been adopted to prepare Pt-Graphene composites. Sequential
reduction involves the separate reduction of graphene from graphene oxide and platinum from its
precursor salt and the subsequent preparation of the composite. An alternate reduction method
involves the simultaneous reduction of both metal nanoparticles and the graphene oxide
[12][13][14]. In the microwave assisted synthesis methods, irradiation helps heating of the
reaction mixture uniformly and rapidly, allowing large-scale and efficient production of
graphene–metal composites [15].
Other techniques for metal nanoparticle decoration on the graphitic nanostructure include
electro-deposition [16], photochemical [17], and solventless bulk synthesis [18]. Although these
methods present some advantages over solution-based techniques, they are expensive and energy
consuming.
In this study, a single step modified polyol method has been employed to disperse Pt
nanoparticles on reduced graphene oxide using H2PtCl6 as a Pt precursor, and ethylene glycol
and NaBH4 as a reducing agent for Pt precursor and graphene oxide, respectively. This simpler
one-pot synthesis approach has been adopted to obtain a uniform dispersion of platinum
nanoparticles on the graphene support [7].](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-10-2048.jpg)
![11 | P a g e
4. Experimental Section
4.1 Materials. Graphite, H2PtCl6, NaBH4 and were obtained from Alfa-Aeser while KMnO4,
NaNO3, C2H6O2, H2O2 and were obtained from Merck. Nafion and N2H4-H2O was obtained from
Sigma Aldrich.
Graphite oxide (GO) was prepared by a modified Hummer’s method (Figure 2) [19]. In brief,
this method involves vigorous stirring of a mixture of graphite powder and sodium nitrate with
concentrated sulfuric acid followed by oxidation by potassium chlorate. The resulting solution is
then washed with deionized water, subject to several cycles of centrifugation and dried to obtain
graphite oxide flakes. This is stored and dispersed in solvents as needed.
Figure 2. Schematic diagram of Hummer’s method [21]
4.2 Synthesis of Pt/graphene nanocomposite.50mg of graphite oxide obtained from the
modified Hummer’s method was dispersed in ethylene glycol (1mg/ml). It was then
ultrasonicated for one hour to exfoliate the graphite oxide to graphene oxide. A homogeneous
graphene oxide slurry was obtained. Subsequently, 120mg of H2PtCl6.6H2O was dissolved in the
graphene oxide slurry and sonicated. The pH of the resultant solution was adjusted to 10 by the
addition 100mg of NaOH and stirring vigorously. The solution was transferred to a round bottom
flask and the temperature was increased up to 120˚C. When the reaction temperature reached
120˚C, 2ml of NaBH4 (20mg/ml) was added dropwise and refluxed at 120˚C for 1 hour. After
complete reduction of H2PtCl6.6H2O to platinum, the solution is cooled and neutralized using 0.1
M HCl (aq) so as to obtain a pH of 7, washed and centrifuged 3 times with water.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-11-2048.jpg)

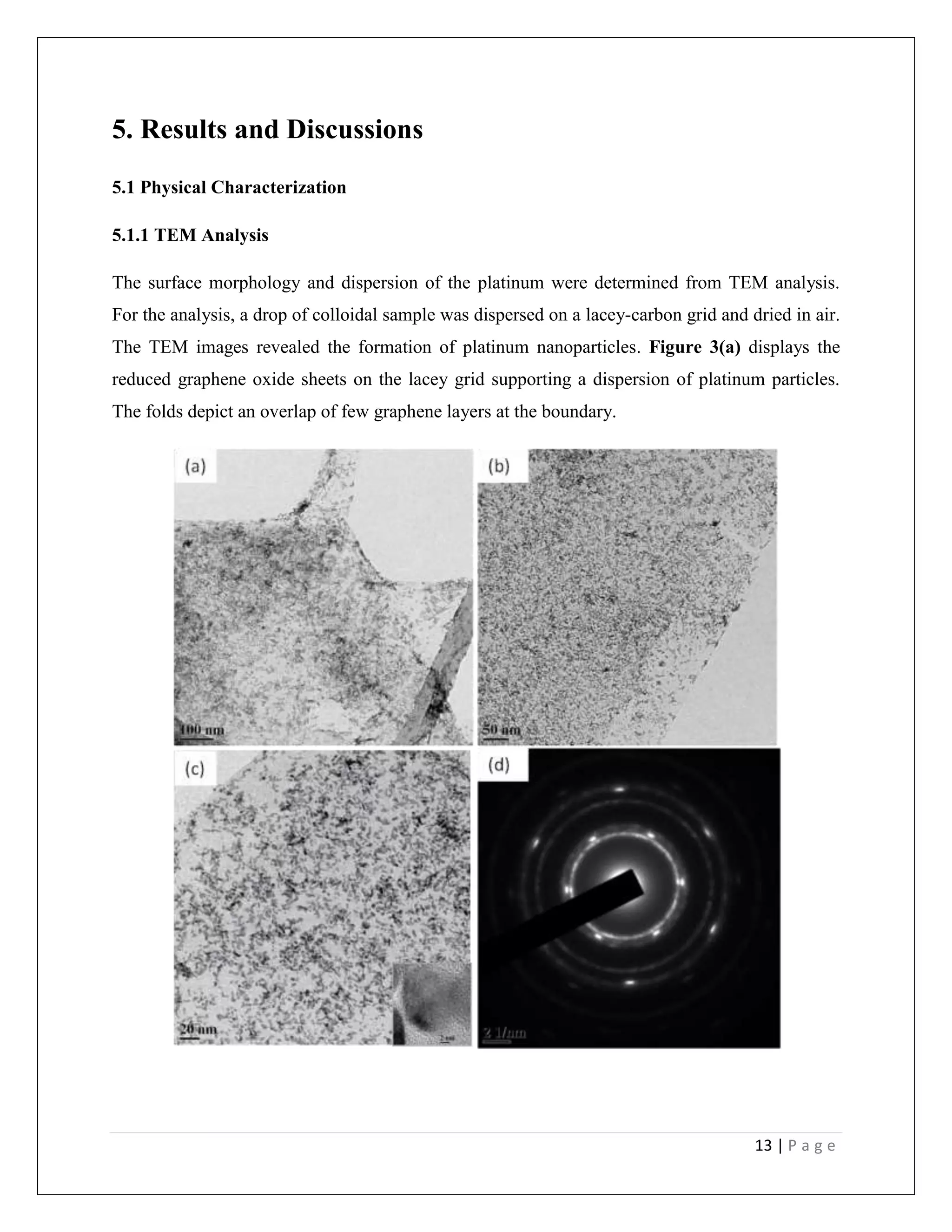

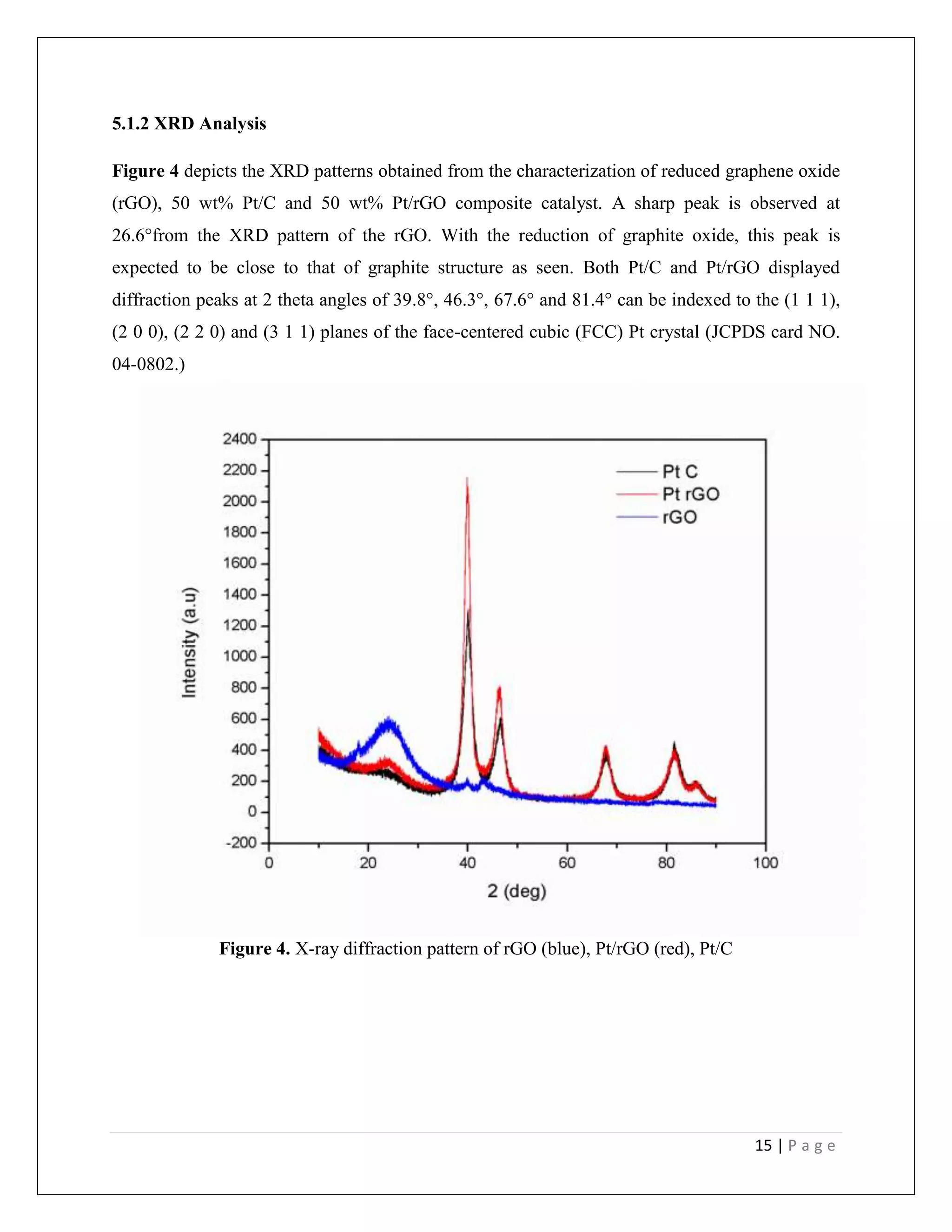

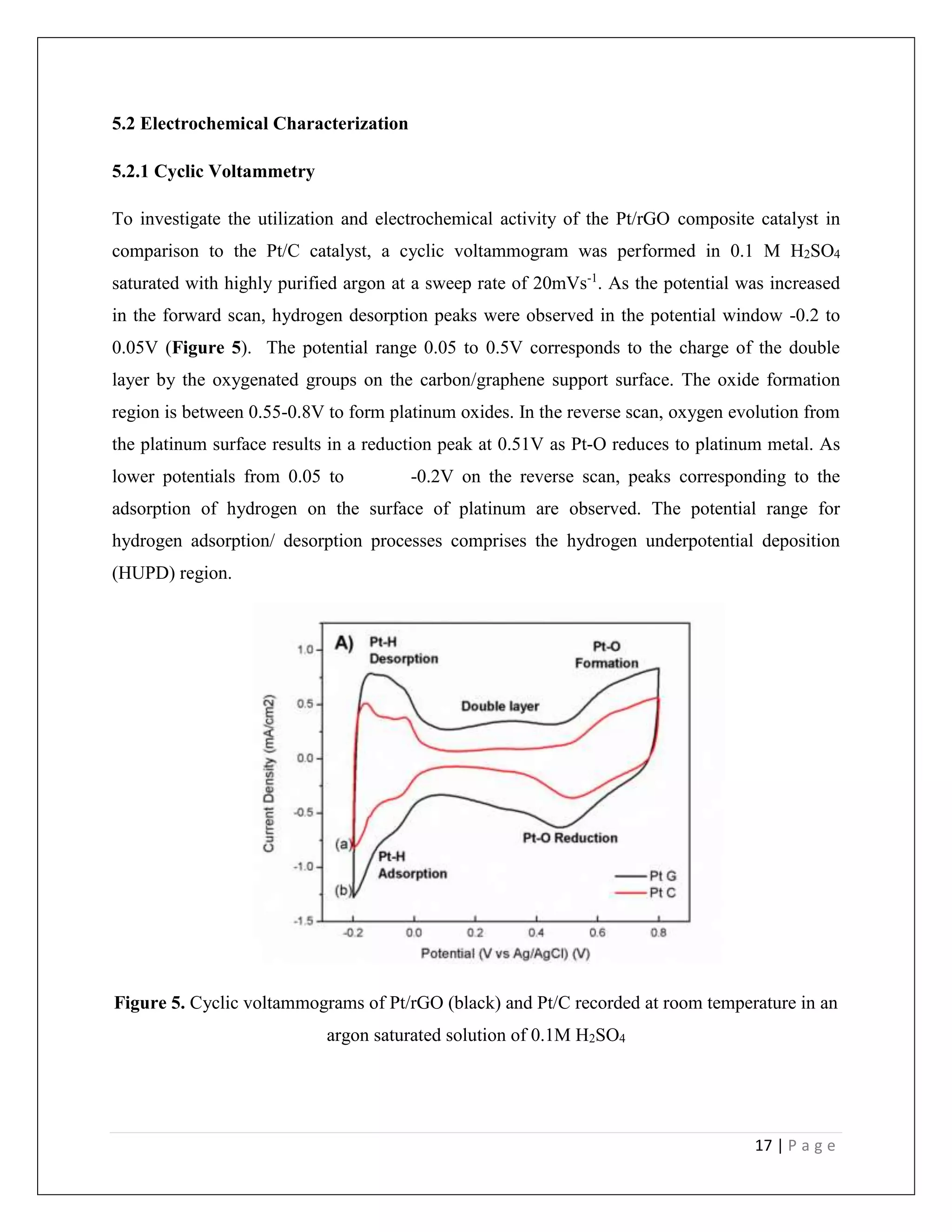
![18 | P a g e
A clear distinction was observed in the voltammograms of Pt/C and Pt/rGO. The double layer
current in the potential regime 0.05-0.5V in the case of the Pt/rGO composite is significantly
higher as when compared with conventional Pt/C (with equal Pt loading). Graphene is known to
display a high interfacial capacitance partly due to its large specific surface area. The enhanced
double layer consequently renders a higher HUPD current.
Electrochemically active Surface Area: The electrochemically active surface area (ECSA) is
an important parameter that provides information about the number of available active sites. It
accounts not only for the catalytic surface area available for charge transfer but also the access of
a conductive path for electron transfer between the catalyst and the electrode surface. Hydrogen
adsorption/desorption region in an electrochemical system is commonly used to evaluate the
ECSA. The area under the curve is a measure of the hydrogen desorbed, which provides an
estimate of the ECSA.
Equation2 below is commonly employed to calculate the effective surface area.
Equation2. ECSA [cm2
Pt/g Pt] = charge [Qh μC/cm2
]/ (210 [ μC/cm2
]*electrode loading
[gPt/cm2
])
QH- average charge integrated from the voltammogram of the adsorbtion/desorbtion
hydrogen process on the CV curve (mC)
constant 210 shows the charge in theoretical calculation to oxidize a single hydrogen
layer adsorbed on bright platinum (mC)
mPt is the platinum loading on the surface sample (g cm−2
)
The ESCA of the Pt/rGO composite was calculated to be 61.67 m2
/g Pt while that of Pt/C was
35.51 m2
/g Pt. This result indicates a smaller particle size and a far better utilization of Pt in the
Pt/rGO nanocomposites which is essential for improving the practical performance of the
PEMFCs.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-18-2048.jpg)

![23 | P a g e
7. References
[1] K. Sasaki, H. Naohara, Y. Choi1, Y. Cai, W. Chen, P. Liu & R.R. Adzic, Nat. Commun,
2012, 3, 1.
[2] S.Sharma, B.G.Pollet, J. Power Sources, 2012, 208, 96.
[3] B.Fang, N.K. Chaudhari, M.S. Kim, J.H. Kim, J.S.Yu, J. AM. CHEM. SOC., 2009, 131,
15330.
[4] Y. Shao, G. Yin , Y. Gaoa, J. Power Sources, 2007, 171, 558.
[5] S. Park, R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217.
[6] C. Su1, M. Acik, K. Takai, J. Lu, S. Hao, Y. Zheng, P. Wu, Q. Bao, T. Enoki, Y. J. Chabal
& K.P. Loh, Nat. Commun, 2012, 3, 1
[7] H.W. Ha, I.Y. Kim, S.J. Hwang, R.S. Ruoff, Electrochem. Solid-State Lett., 2011, 14, B70.
[8] N.Soin, S.S. Roya, T.H. Limc, J.A.D. McLaughlina, Mater. Chem. Phys., 2011, 129, 1051.
[9] A. Grinou, Y.S. Yun, S.Y. Cho, H.H. Park, H.J. Jin, Materials, 2012, 5, 2927.
[10] R. I. Jafri, N. Rajalakshmi, S. Ramaprabhu, J. Mater. Chem, 2010, 20, 7114.
[11] M.H. Seo, S.M. Choi, H.J. Kim, W.B Kim, Electrochem. Commun., 2011,13,182.
[12] P. Kundu , C. Nethravathi , P.A. Deshpande , M. Rajamathi , G. Madras , N. Ravishankar ,
Chem. Mater., 2011, 23, 2772.
[13] S. Wanga, S.P. Jiang, X. Wanga, Electrochim. Acta, 2011, 56, 3338.
[14] S. Sharma , A. Ganguly , P. Papakonstantinou , X. Miao , M.Li, J. L. Hutchison, M.
Delichatsios, S. Ukleja, J. Phys. Chem. C, 2010, 114 (45), 19459.
[15] B. F. Machadoab, P. Serp, Catal. Sci. Technol., 2012, 2, 54.
[16] T. Maiyalagan, X. Dong, P.Chen, X.Wang, J. Mater. Chem., 2012, 22, 5286.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-23-2048.jpg)
![24 | P a g e
[17] X. Huang, X. Zhou, S. Wu, Y. Wei, X. Qi, J. Zhang, F. Boey, H. Zhang, Small, 2010, 6, No.
4, 513.
[18] Y. Lin, K.A. Watson, M. J. Fallbach, S. Ghose, J.G. Smith Jr., D.M. Delozier, W. Cao, R. E.
Crooks, J. W. Connell, ACS Nano, 2009, 3, 871.
[19] W. Hummers, Jr ., R . Offeman, J. Am. Chem. Soc., 1958, 1339.
[20] Chemistry of Electronic Materials, Andrew R. Barron
[21] M.J. McAllister, J.-L. Li, D.H. Adamson, H.C. Schniepp, A.A. Abdala, J. Liu, M. Herrera-
Alonso, D.L. Milius, R. Car, R.K. Prudhomme, I.A. Aksay, Chem. Mater., 2007, 19, 18, 4396.](https://image.slidesharecdn.com/77e19854-d6f9-4cf3-a067-fefb5688906b-161214010805/75/Final-Report-Graphene-supported-platinum-nanoparticles-1-24-2048.jpg)