To calculate a protein's net charge:
1. Determine the proportion of each ionizable amino acid and terminus that is ionized at a given pH using the amino acid's dissociation constant.
2. Multiply this proportion by the number of each amino acid/terminus to determine its individual charge contribution.
3. Add the contributions from positively charged amino acids and subtract contributions from negatively charged amino acids to calculate the net charge.


![Dissociation Constant
• The association and dissociation of the H+
with the amino acid
is governed by its dissociation constant, KD.
• Dissociation constant is the ratio of the concentration of the
dissociated forms to the concentration of the combined form.
– Each amino acid has a separate dissociation constant.
– The equation below uses [R-
] to indicate the concentration of the
amino acid when the H+
is dissociated from it.
• The pK value is analogous to pH.
– pK = -log10KD
– KD = 10-pK
][
]][[
RH
RH
KD
−+
=](https://image.slidesharecdn.com/calculatingpi-140318052446-phpapp02/85/Calculating-p-i-4-320.jpg)
![Calculating the
Proportion with H
Associated
• Starting with the dissociation
constant equation, we need to
derive an equation for the
proportion of amino acid
molecules that have H associated
at a given pH.
proportion
RRH
RH
=
+ −
][][
][
][
]][[
RH
RH
KD
−+
=
DK
RH
RH
]][[
][
−+
=
][
]][[
]][[
−
−+
−+
+
=
R
K
RH
K
RH
proportion
D
D
We want:
We rearrange to get:
Substituting this into the
proportion equation:
][]][[
]][[
−−+
−+
+
=
RKRH
RH
proportion
D
DKH
H
proportion
+
= +
+
][
][
Start with the definition of
dissociation constant:
Some algebraic manipulation:
Our final result:](https://image.slidesharecdn.com/calculatingpi-140318052446-phpapp02/85/Calculating-p-i-5-320.jpg)
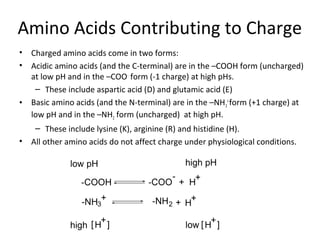
![Acidic and Basic Amino Acids
• The proportion equation calculates the proportion of a given amino acid
that has H associated with it at a given pH.
– Recalling that [H+
] = 10-pH
and pH = -log10[H+
]
• For the basic amino acids (H, K, and R) and the N-terminus, the form with
H associated is –NH3
+
, which has a +1 charge.
– Thus, the contribution to net charge for each of these amino acids is just the
number of each amino acid multiplied by the proportion that has H
associated.
• For the acidic amino acids (D and E) and the C-terminus, the form with H
associated is –COOH, which is uncharged. Thus, the proportion that is in
the charged form ( -COO-
) is 1 minus the proportion with H associated.
– Also, remember that the charged form has a negative charge, The net
contribution from acidic amino acids needs to be subtracted from the
contribution of the basic amino acids.](https://image.slidesharecdn.com/calculatingpi-140318052446-phpapp02/85/Calculating-p-i-6-320.jpg)
