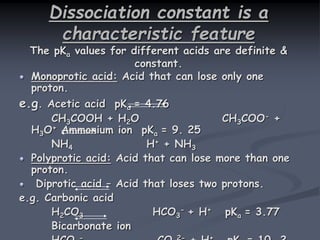
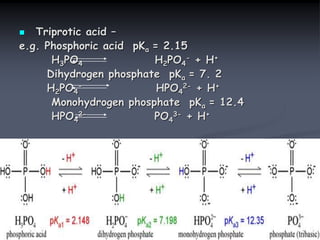
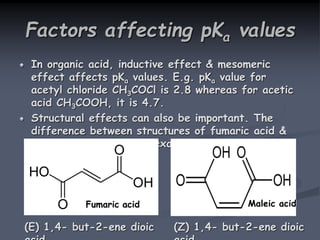
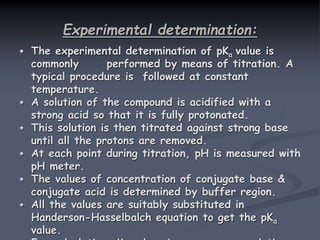
The document discusses the acid dissociation constant (pKa), exploring its definition, theoretical background, and significance in various scientific fields. It highlights the importance of pKa in understanding acid-base equilibria, enzyme activity, and pharmaceutical applications, including the behavior of weak acids and bases. Additionally, it outlines factors influencing pKa values and methods for experimental determination.



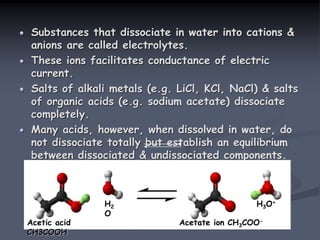
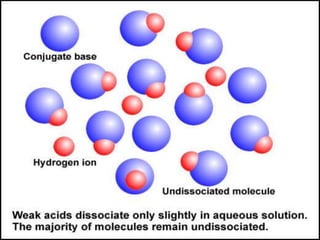
![Water is a weak electrolyte
Water dissociates as follows:
H2O H+ + OH –
In partial dissociation of weak electrolyte H2O,
the constant for above equilibrium equation can be
written as:
Keq = [H+] [OH -]
[H2O]
Where, Keq = physical constant or equilibrium
constant.
At 25 oC the value of Keq for water is very small.
Thus the concentration of water molecules is
almost constant.
Keq x [H2O] = [H+] [OH -]
It is also termed as ionic product of water](https://image.slidesharecdn.com/1-200430161157/85/pka-and-acid-dissociation-constant-8-320.jpg)

![Acid dissociation constant
An acid dissociation constant Ka (also known as acidity
constant or acid ionization constant) is a quantitative
measure of strength of an acid in the solution. It is
the equilibrium constant for a chemical reaction known
as dissociation in the context of acid-base reaction.
The equilibrium can be written symbolically as below:
HA H+ + A –
Here, HA is a weak acid that dissociates into H+
cation & A – anion. Hence, the dissociation constant
may be written as:
Ka = [H+] [A –]
[HA]
A logarithmic measure of Ka is more common &
convenient term used in practice. pKa is the negative
log of Ka & is also referred to as acid dissociation](https://image.slidesharecdn.com/1-200430161157/85/pka-and-acid-dissociation-constant-10-320.jpg)


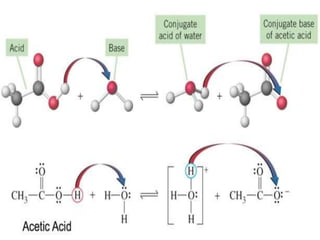
![Handerson-Hasselbalch
EquationIt defines the relationship between, pH, pKa &
concentration of Conjugate acid & Conjugate base.
conjugate acid conjugate base +
H+
At equilibrium,
Ka = [H+] [conjugate base]
[conjugate acid]
Rearranging above equation & taking log on both
the sides, we get,
log 1 = log 1 + log
[conjugate base]
[H+] Ka
[conjugate acid]](https://image.slidesharecdn.com/1-200430161157/85/pka-and-acid-dissociation-constant-14-320.jpg)
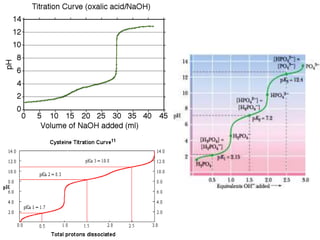
![At half neutralization, when ratio of concentration
of [conjugate base] / [conjugate acid] is 1 : 1 ,
pH equals the pKa of acid as log 1 = 0.
Thus, the pH of the solution can be predicted
when pKa value & concentration of acid & base are
known & conversely it is possible to calculate the
equilibrium constant when pH of the solution is
known.
These calculations find application in many
different areas of chemistry, biology, medicine &
geology.](https://image.slidesharecdn.com/1-200430161157/85/pka-and-acid-dissociation-constant-15-320.jpg)