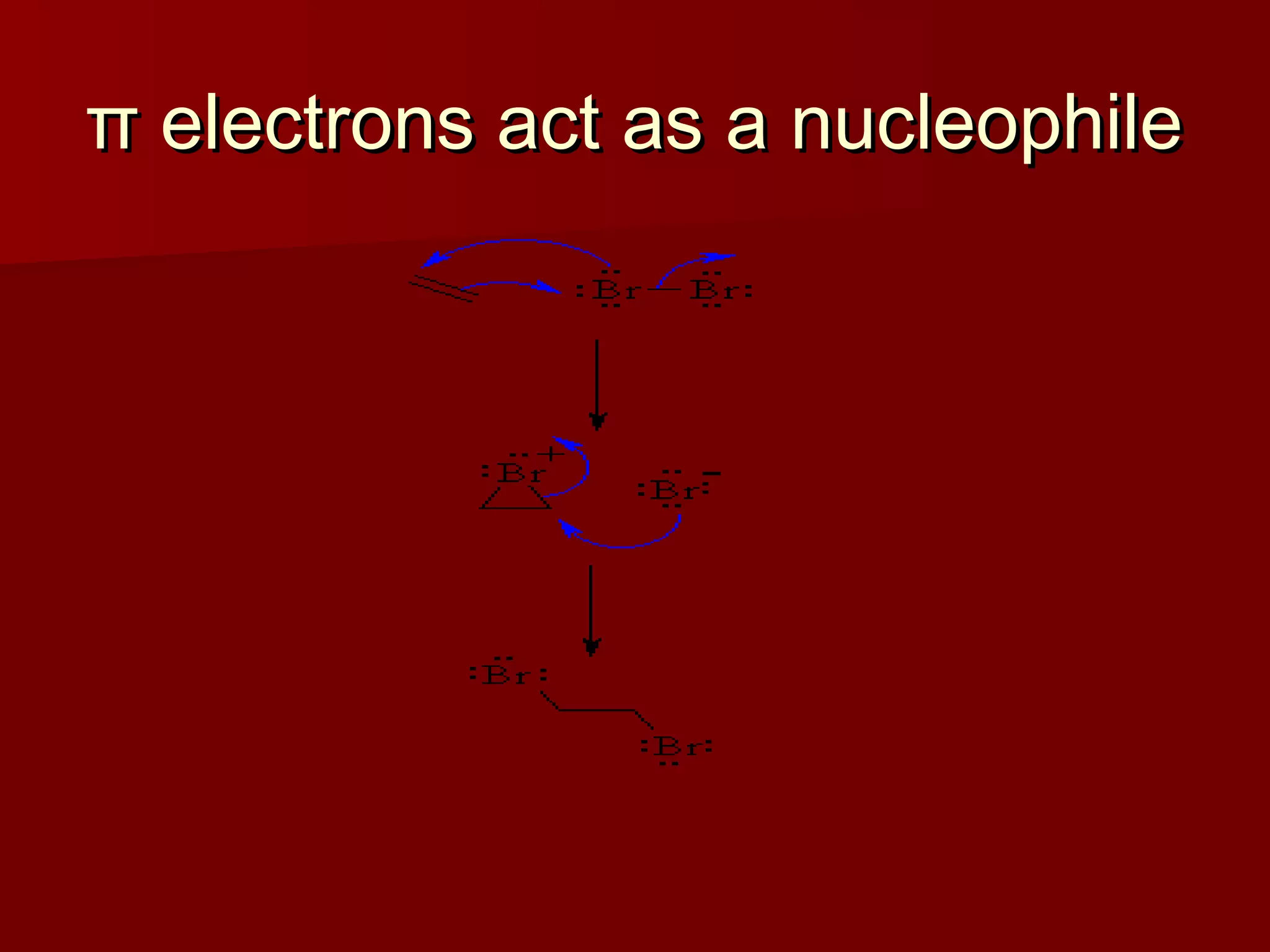
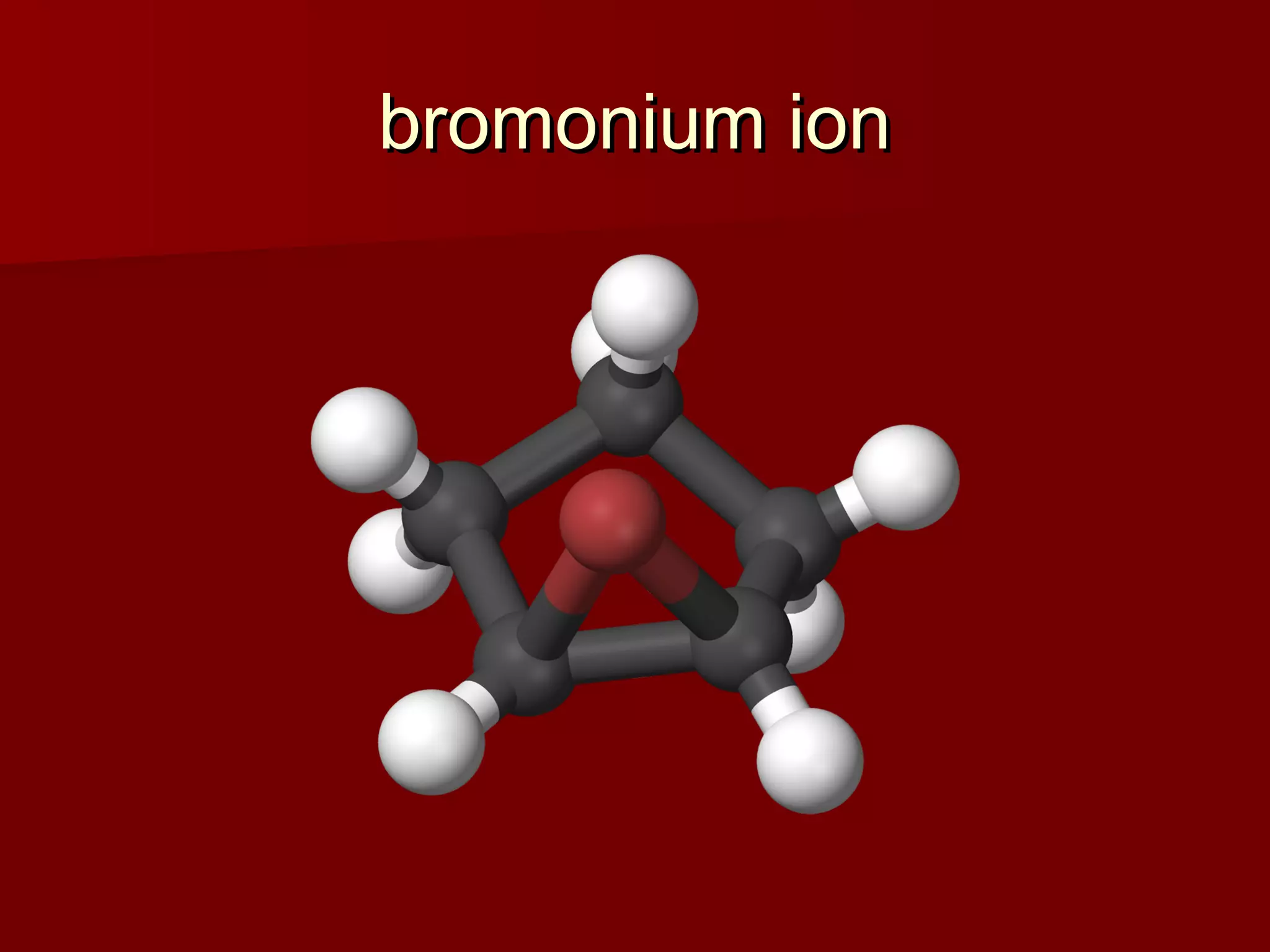
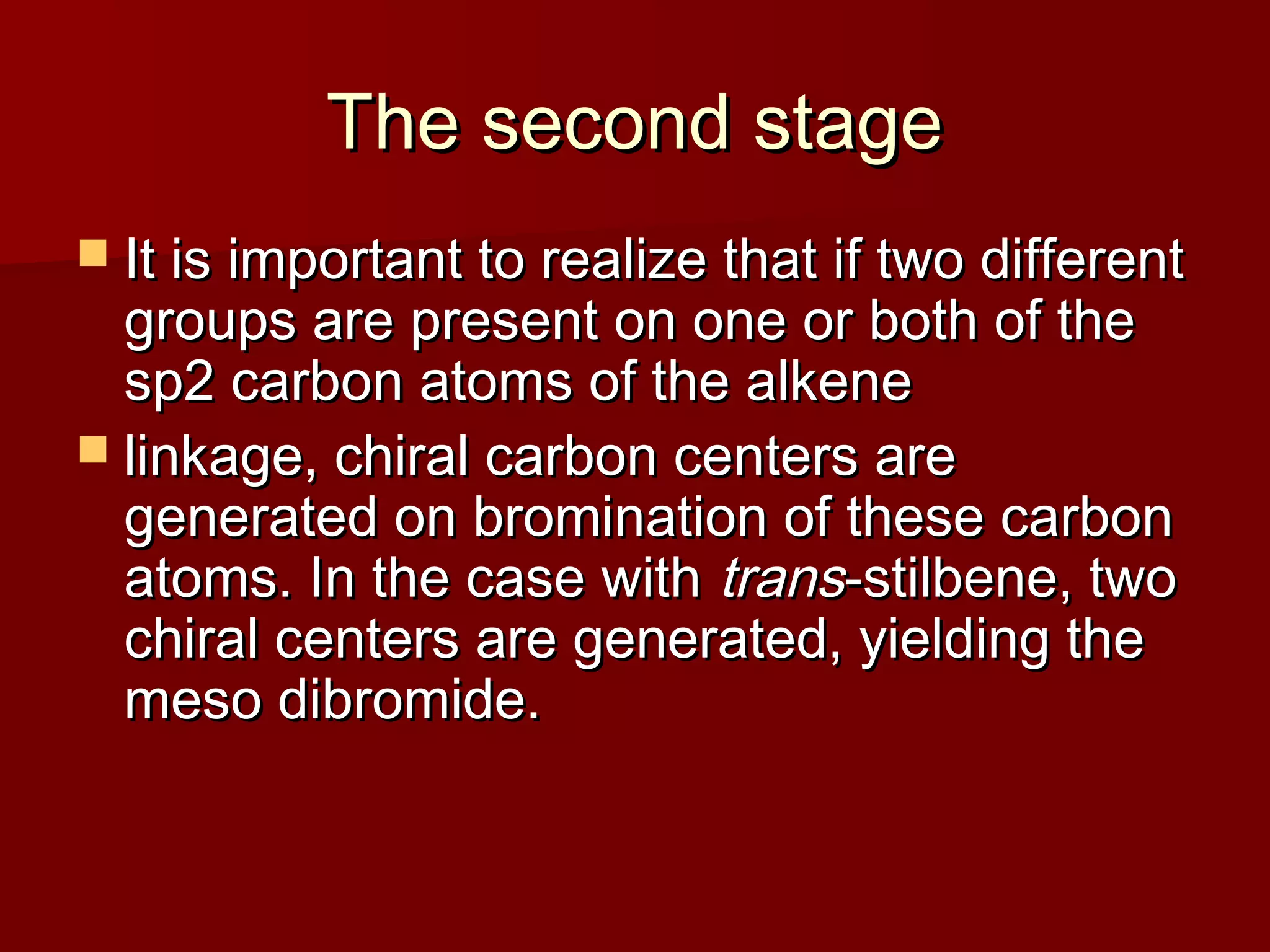
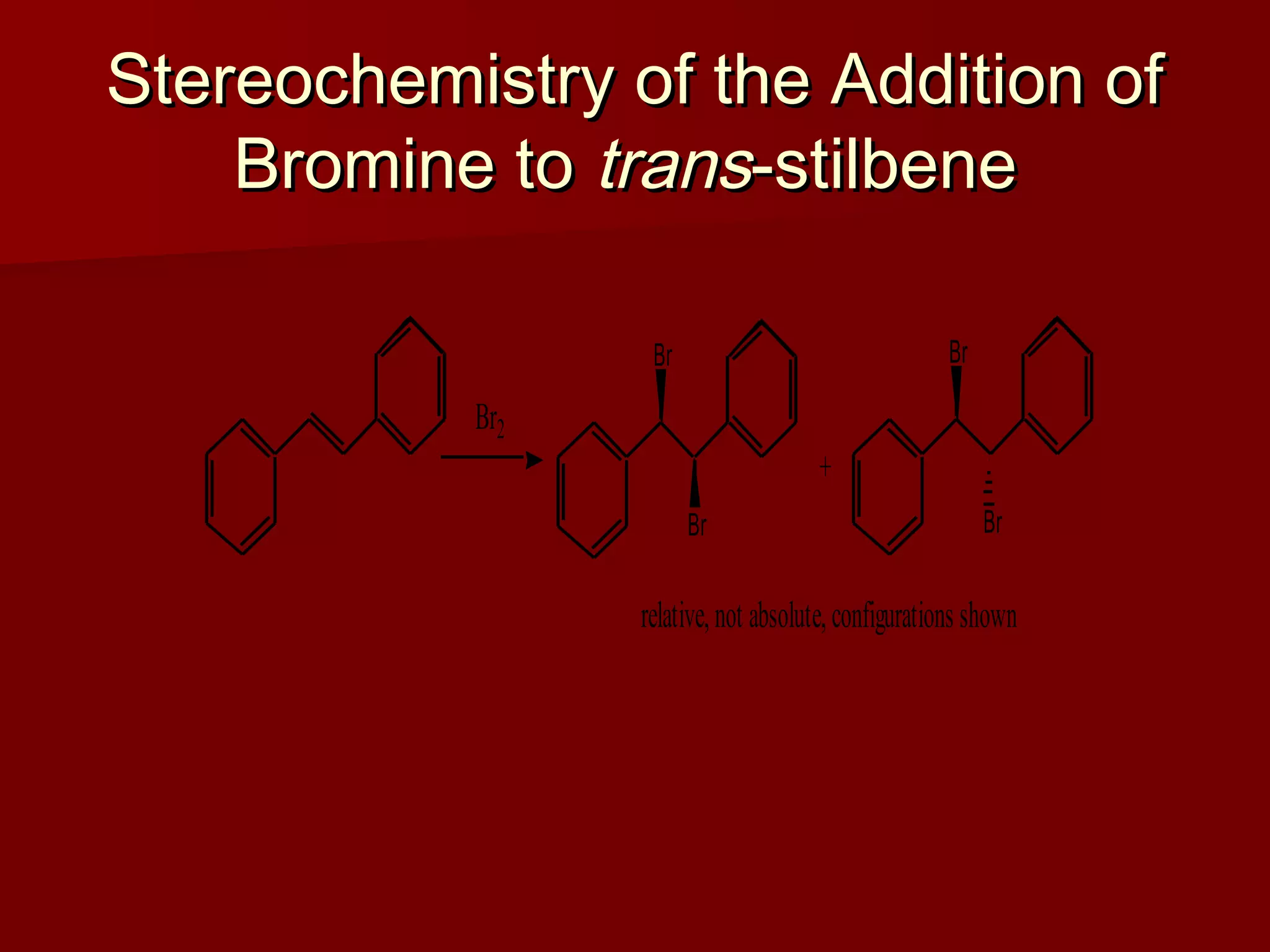
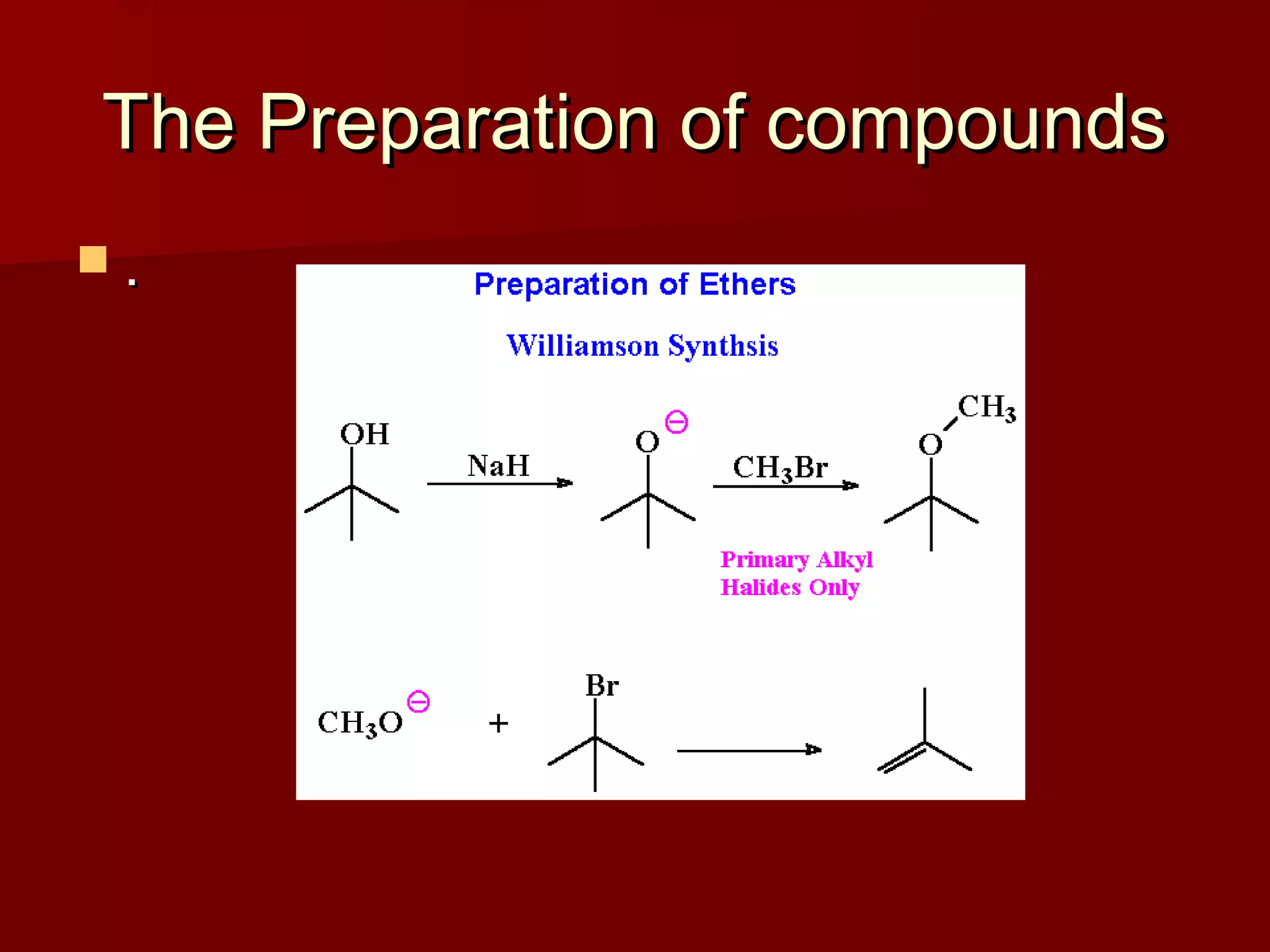
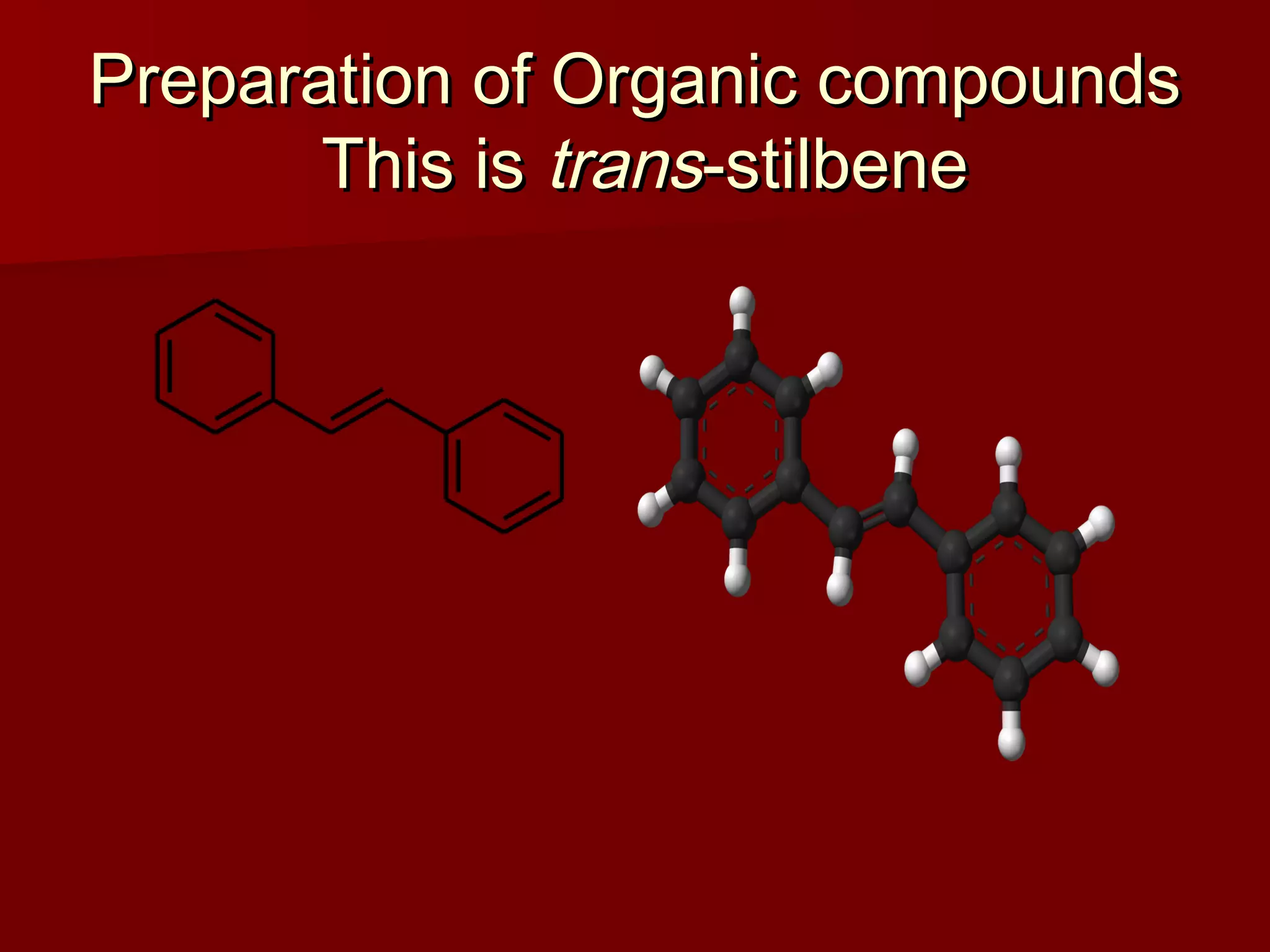
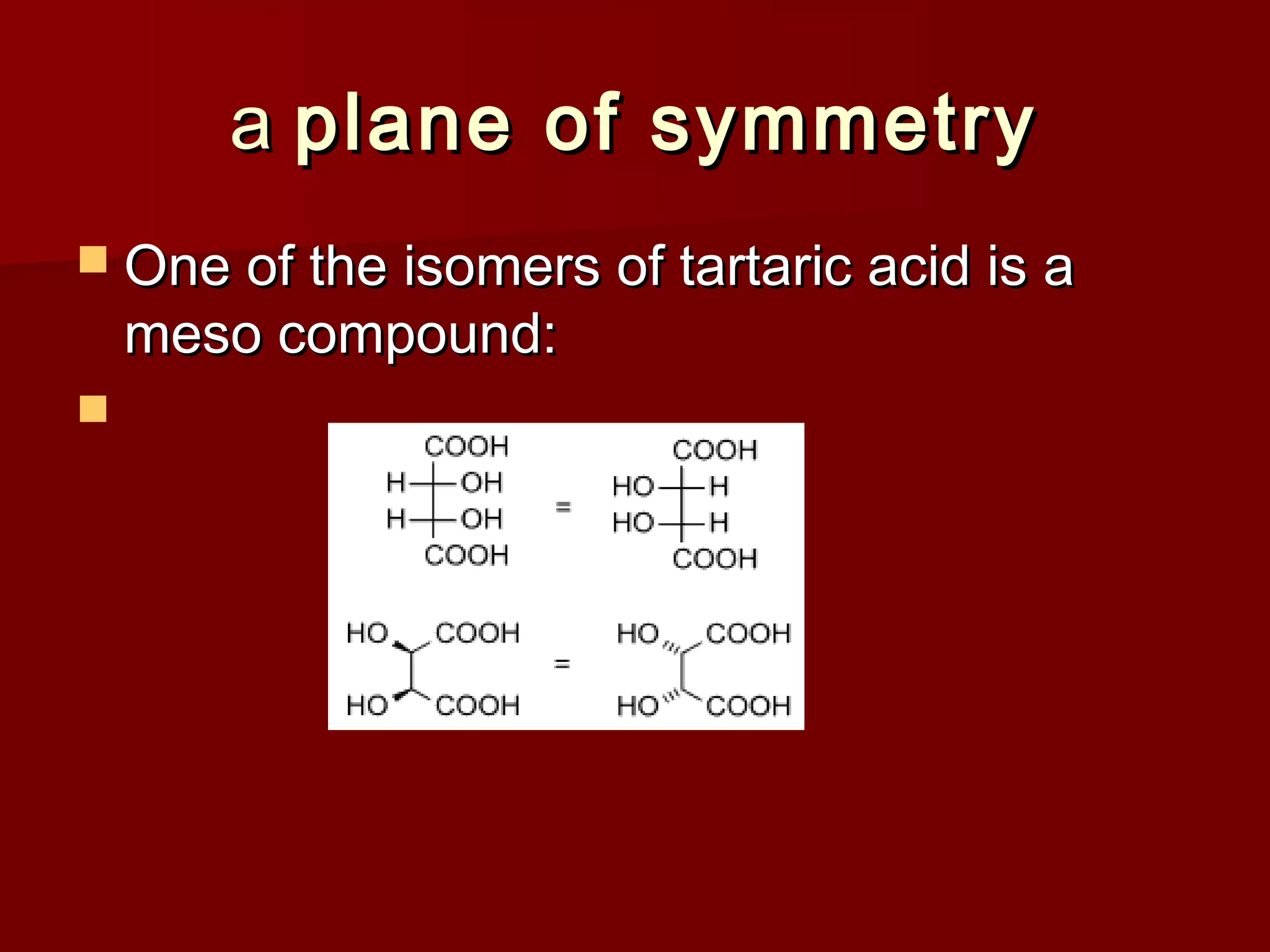
The document describes the bromination of trans-stilbene, which generates two chiral centers and results in either the meso or d,l diastereomer product. It provides details on the stereospecific anti addition reaction using pyridinium bromide perbromide that exclusively forms the meso diastereomer. A microscale procedure is included for brominating trans-stilbene and isolating the meso-stilbene dibromide product through recrystallization.

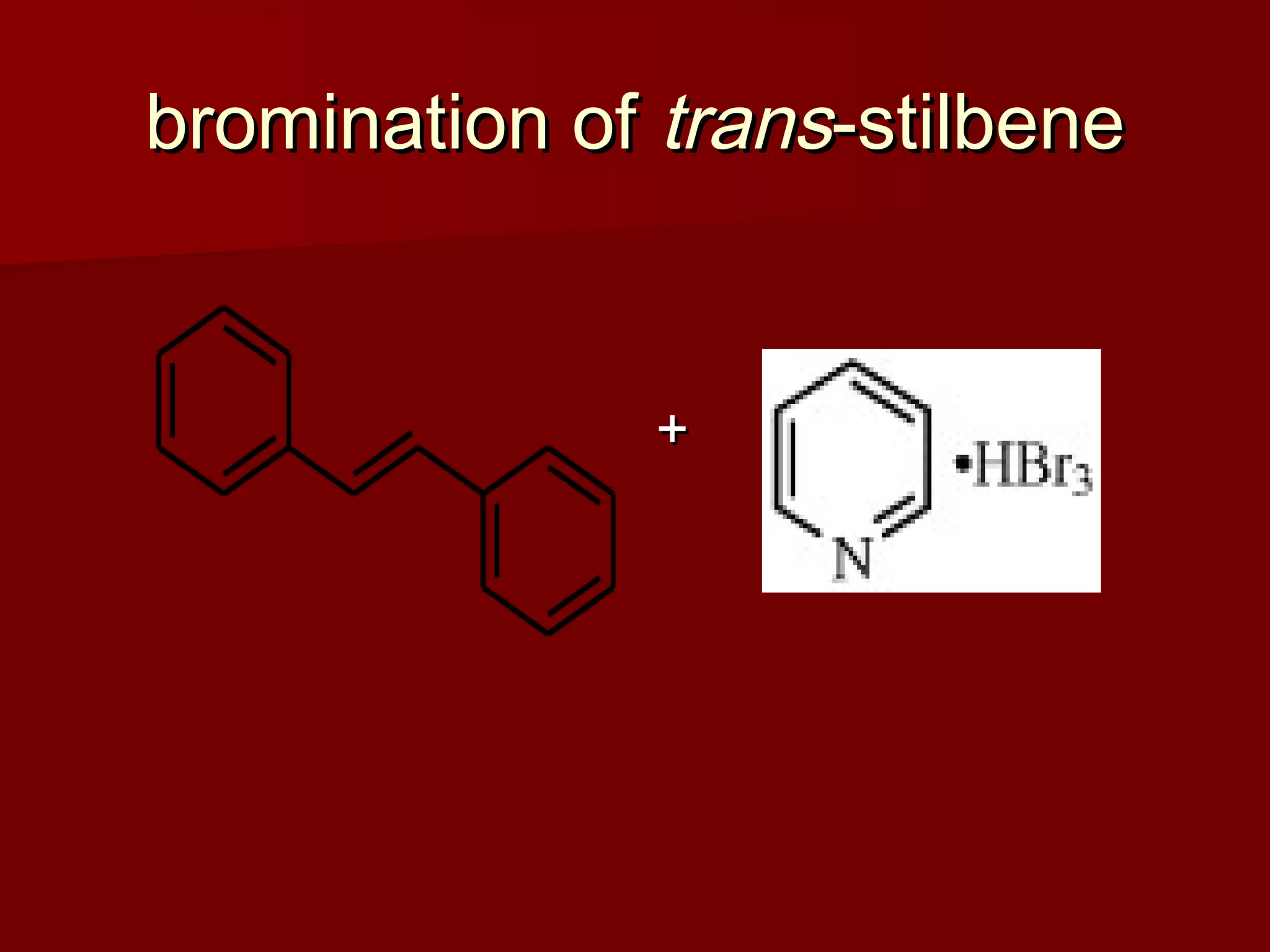

![pyridinium bromide perbromidepyridinium bromide perbromide
CAS [39416-48-3] CAS [39416-48-3] C C55HH66BrBr33N (MW 319.86)N (MW 319.86)](https://image.slidesharecdn.com/brominationofalkenes-101116033113-phpapp01/75/Brominationof-alkenes-7-2048.jpg)