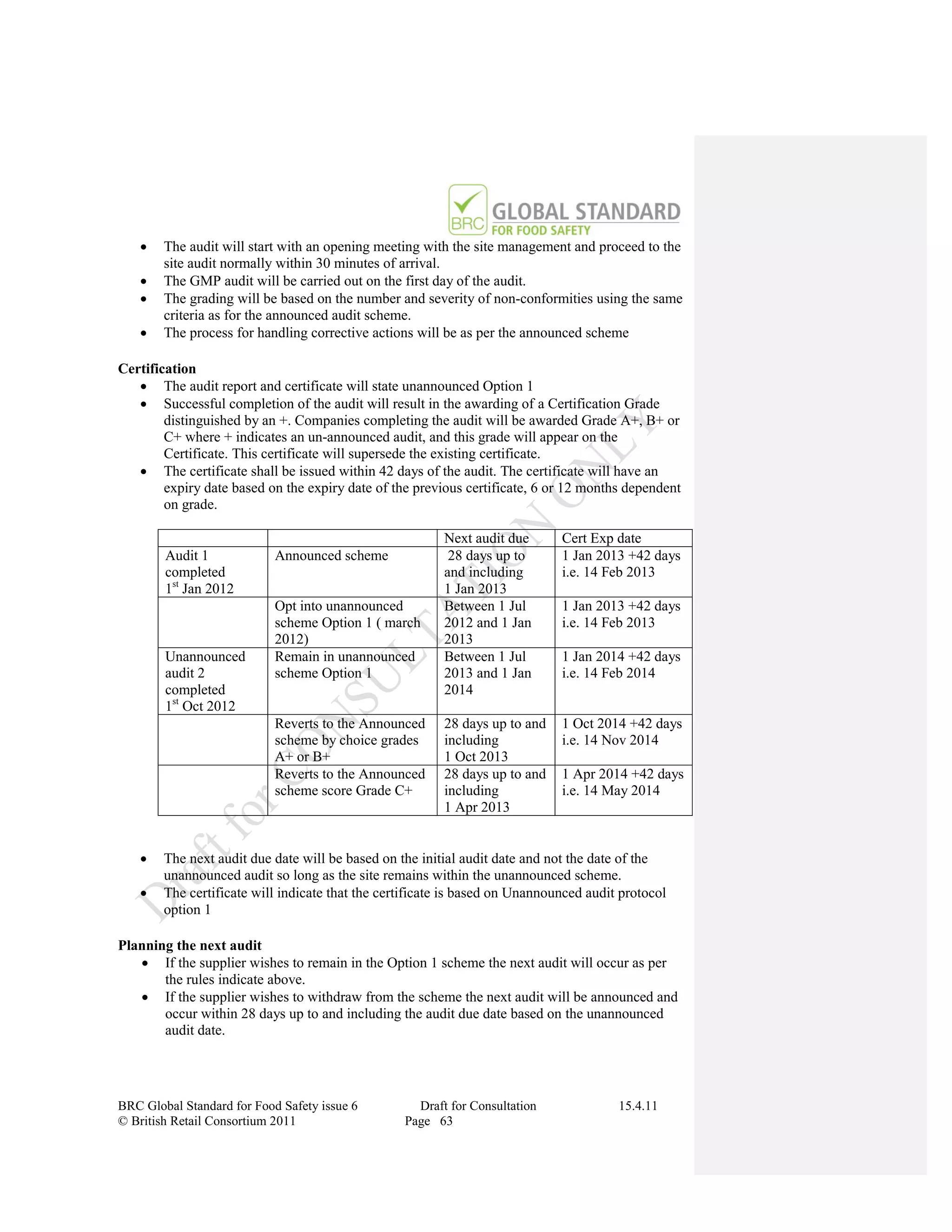
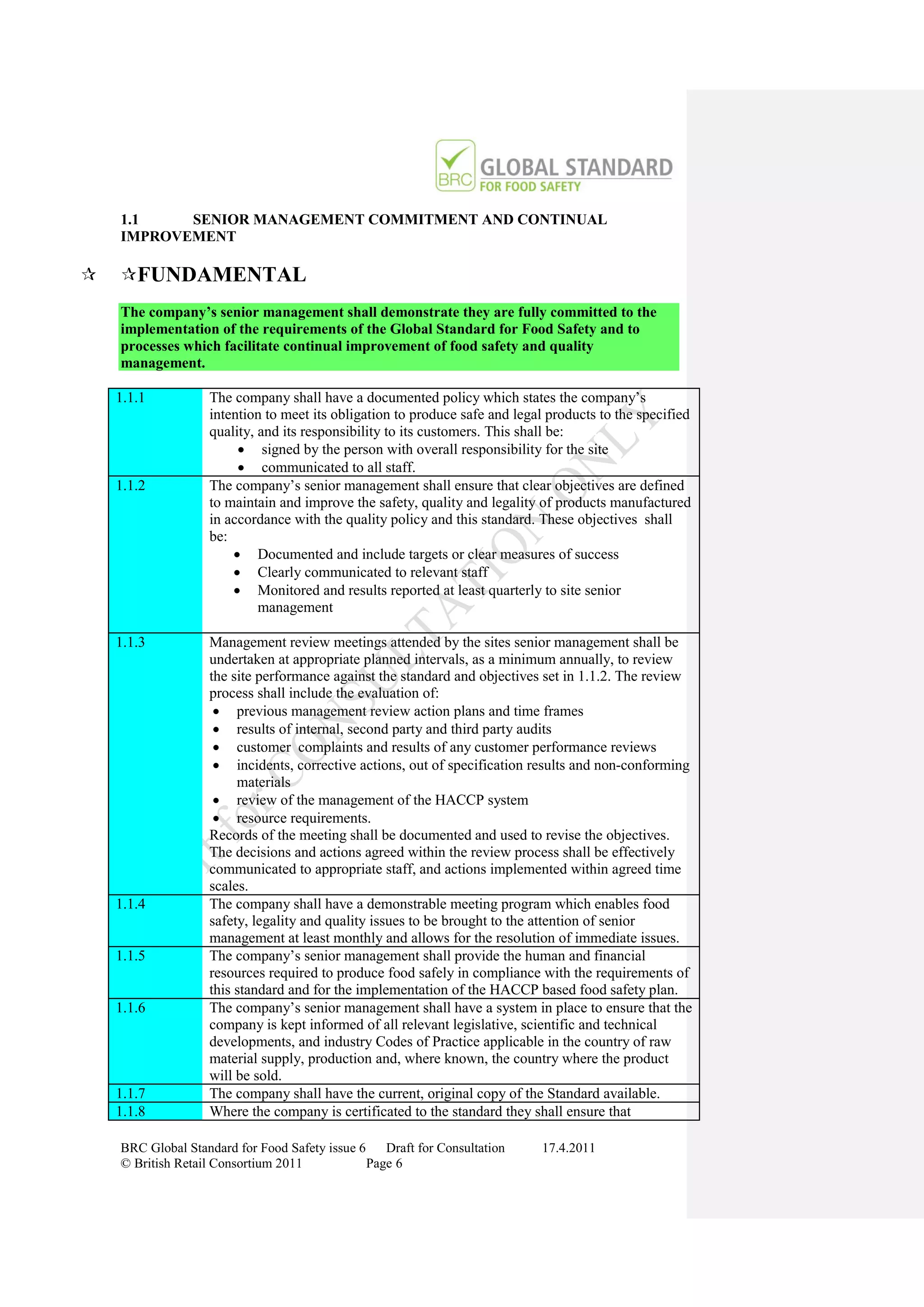
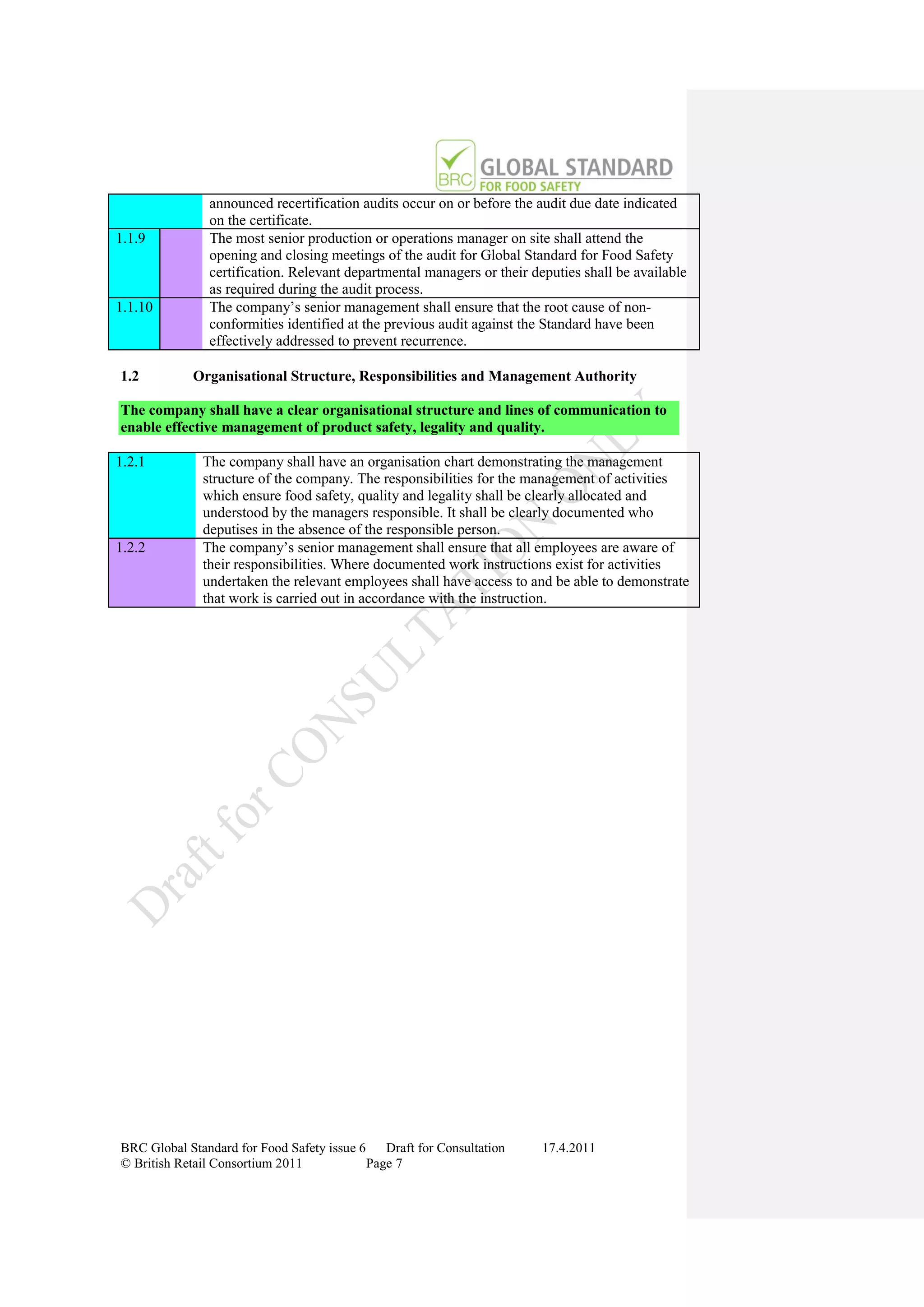
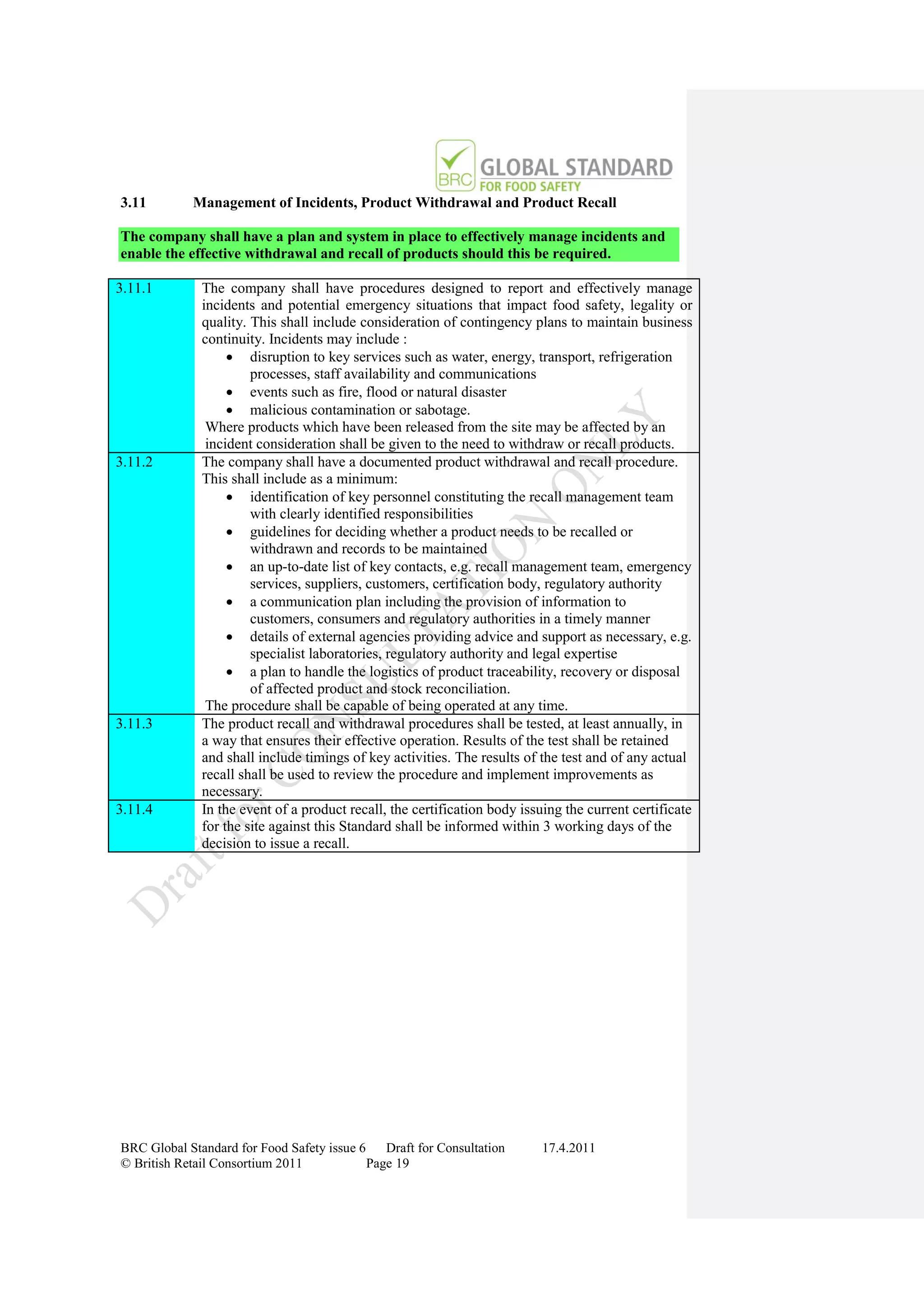
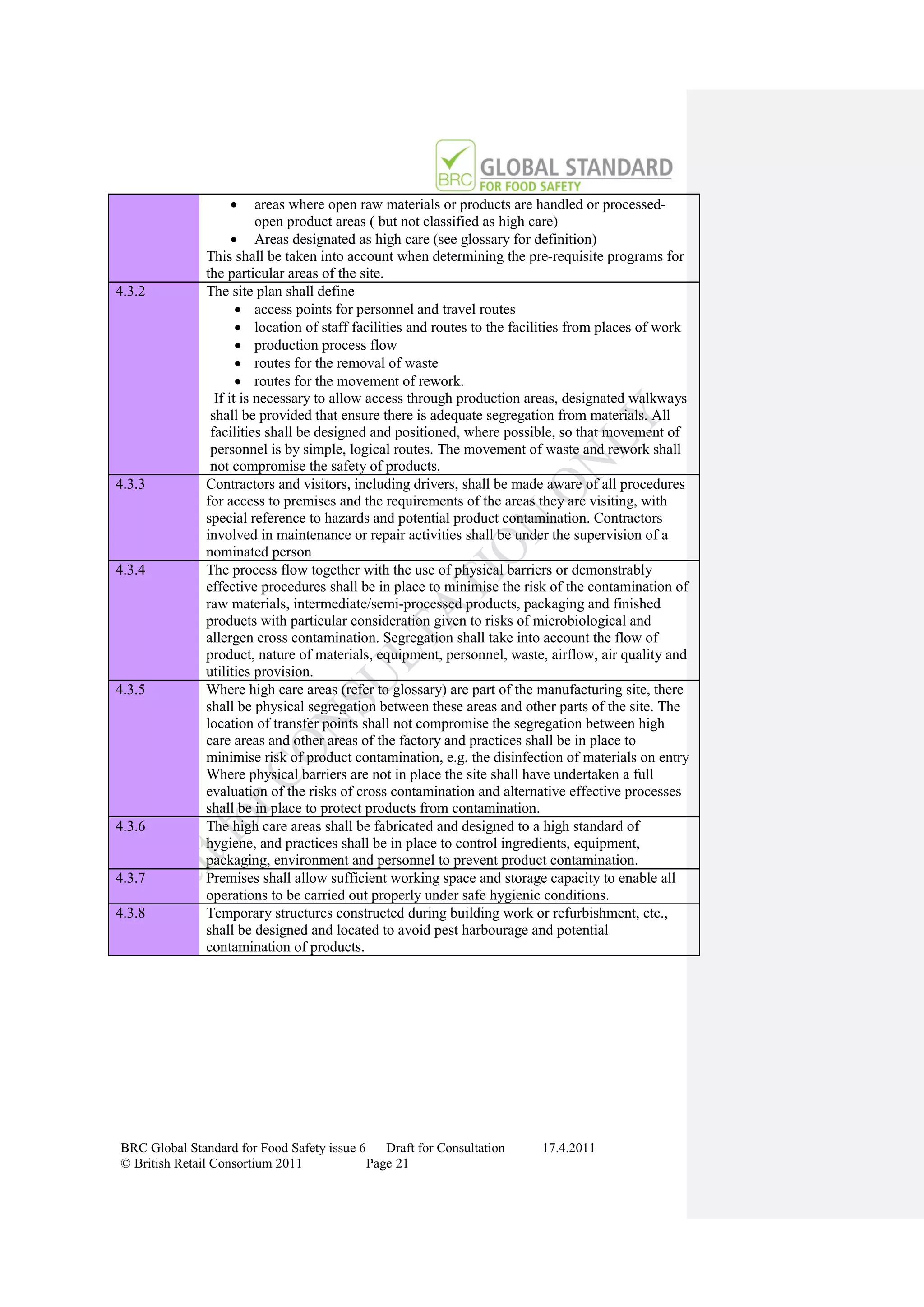
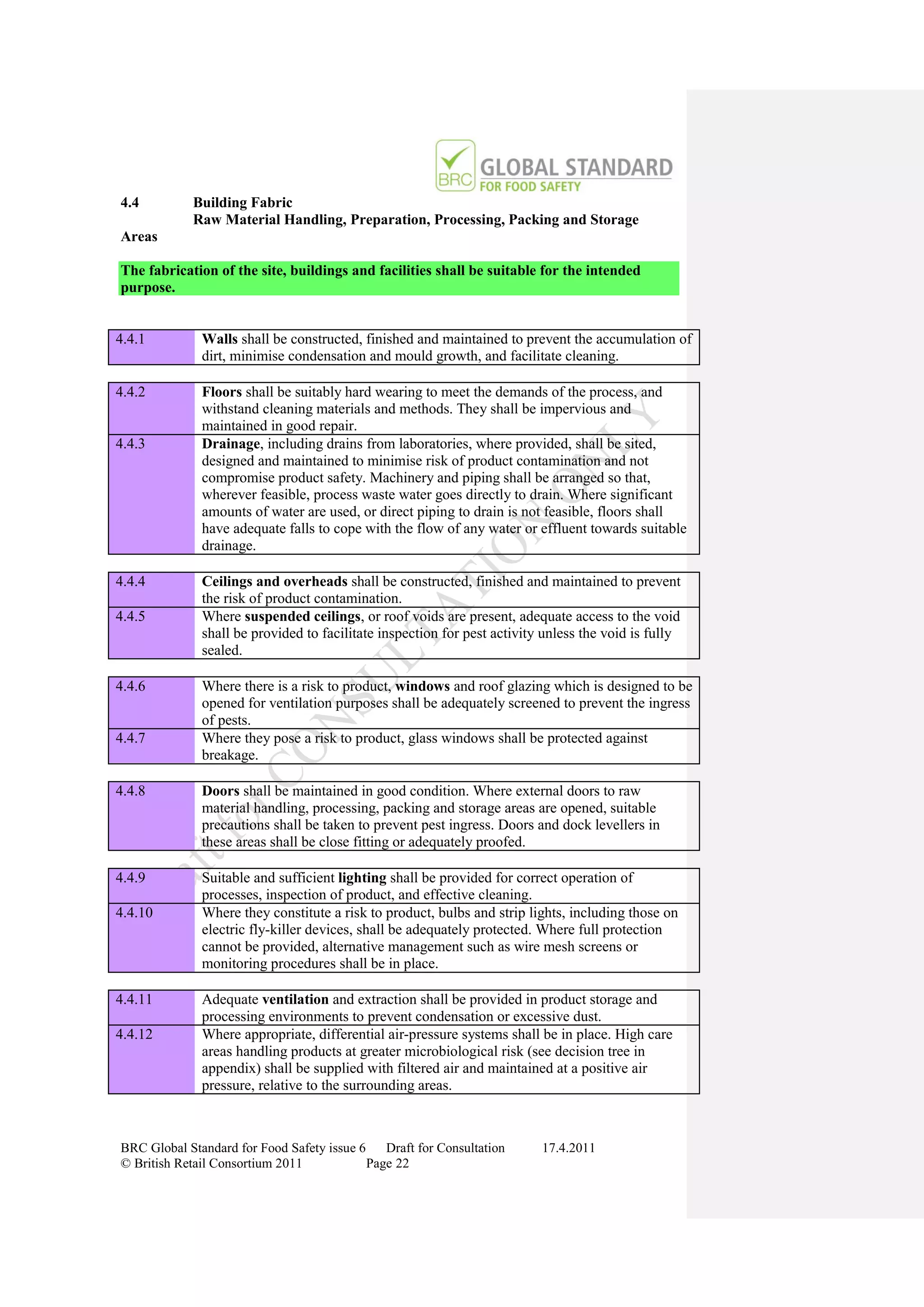
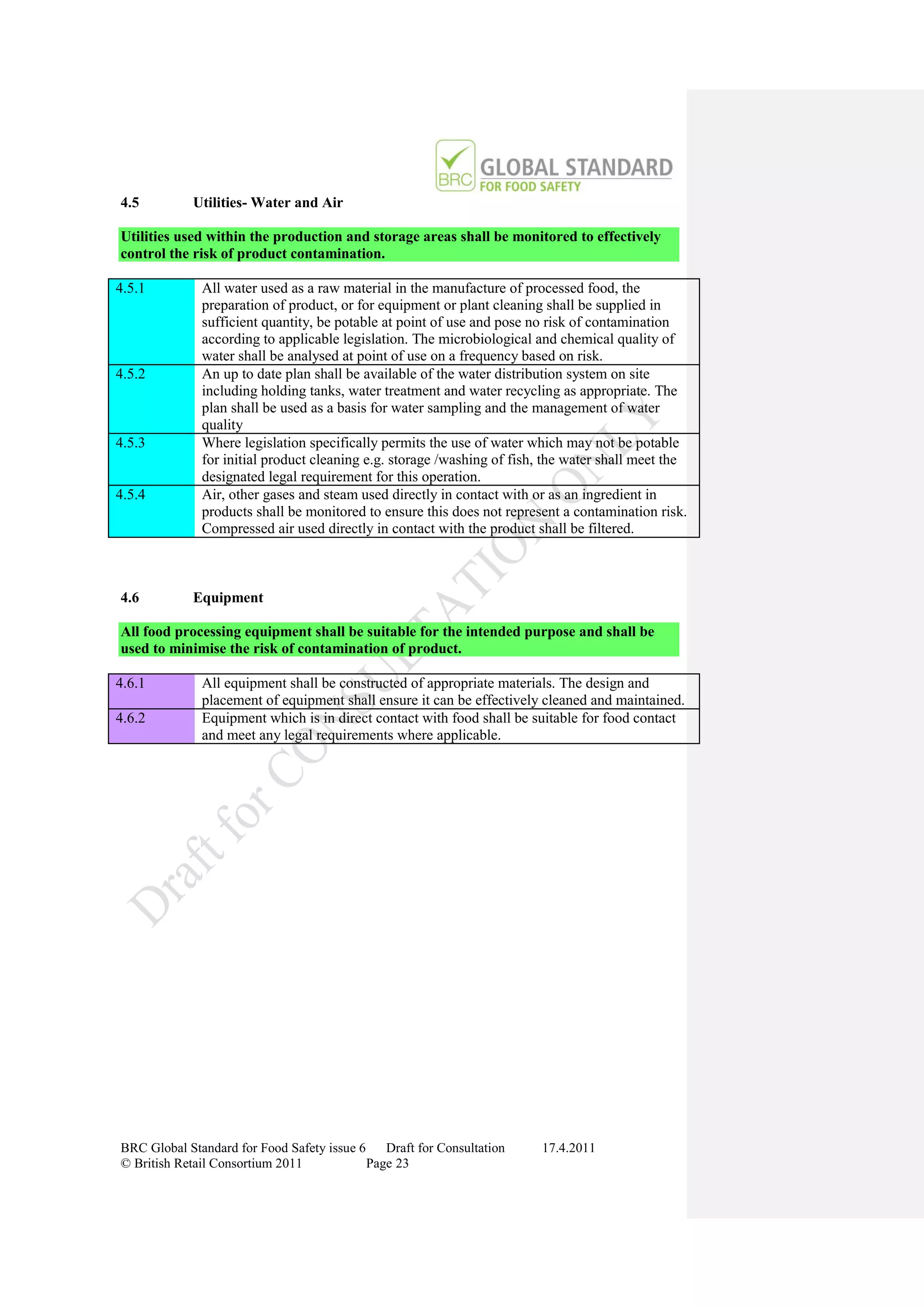
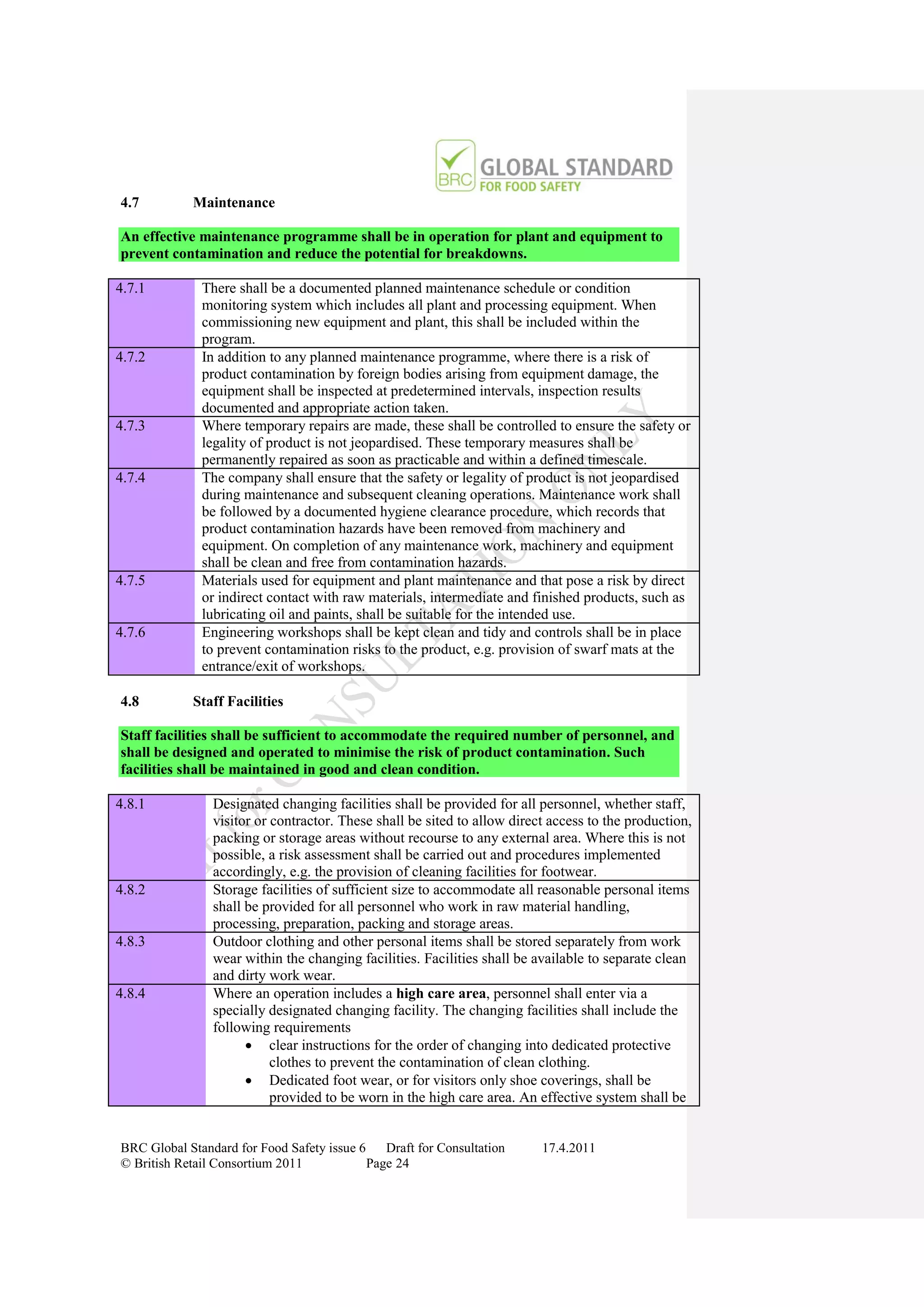
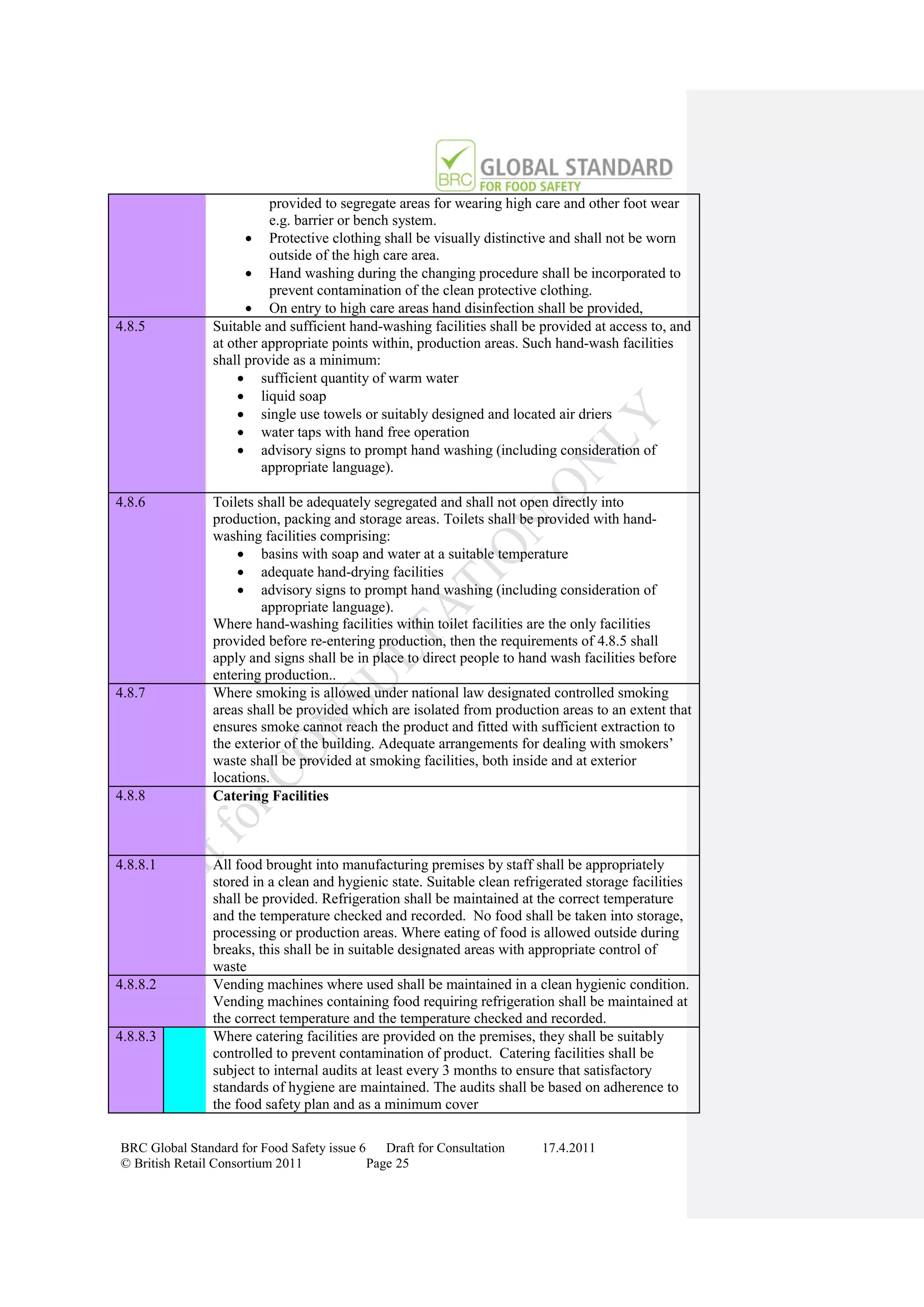
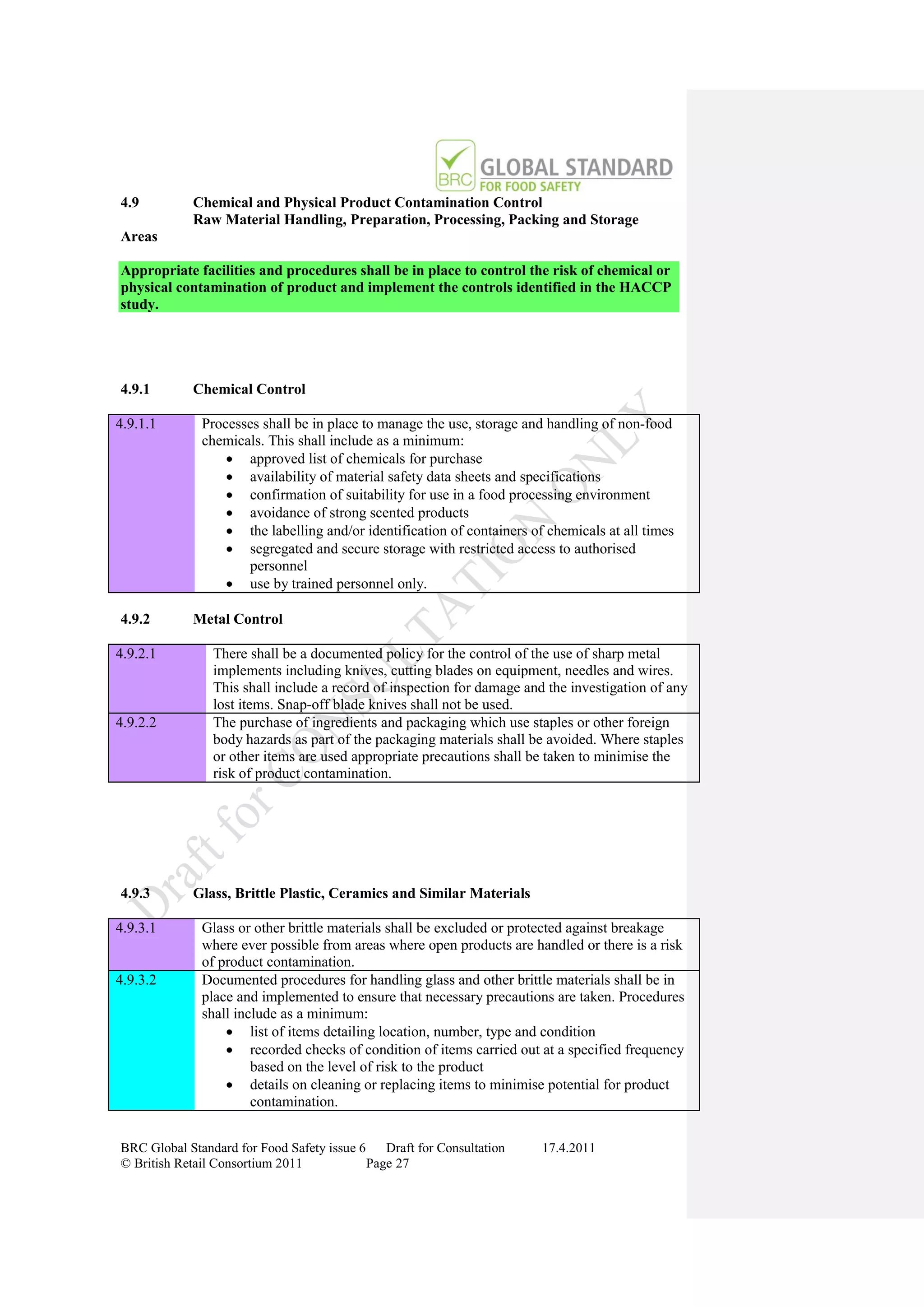
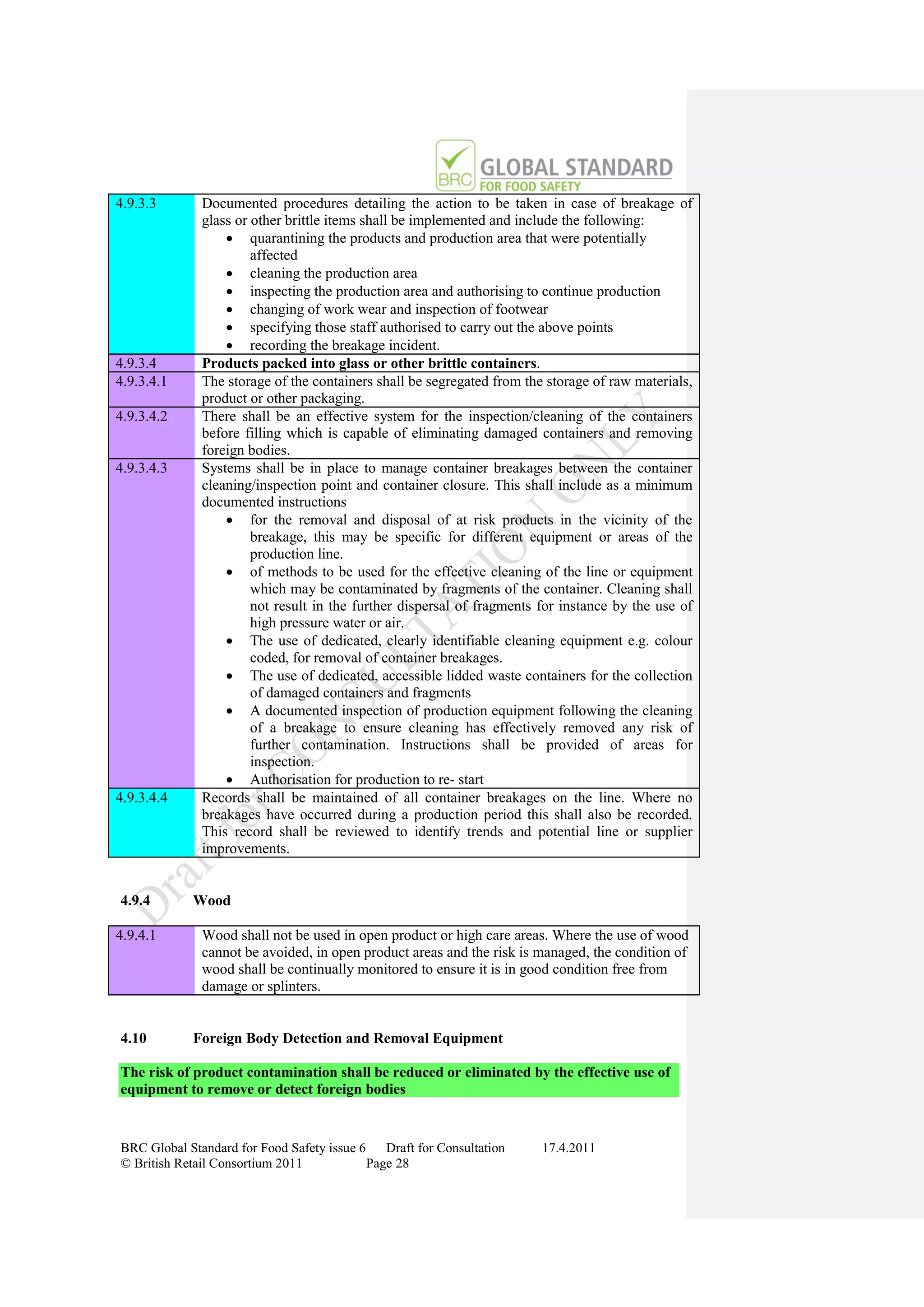
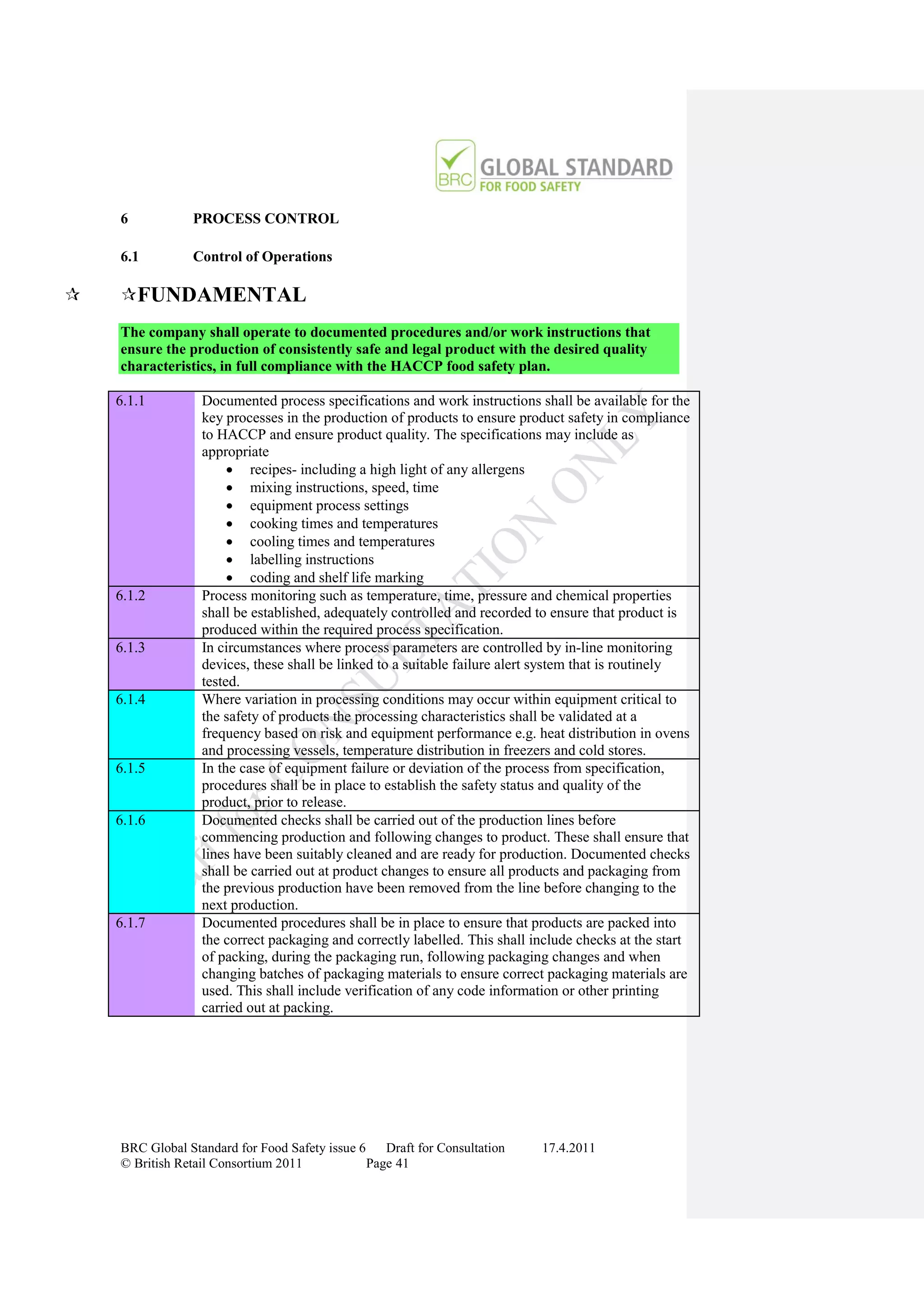
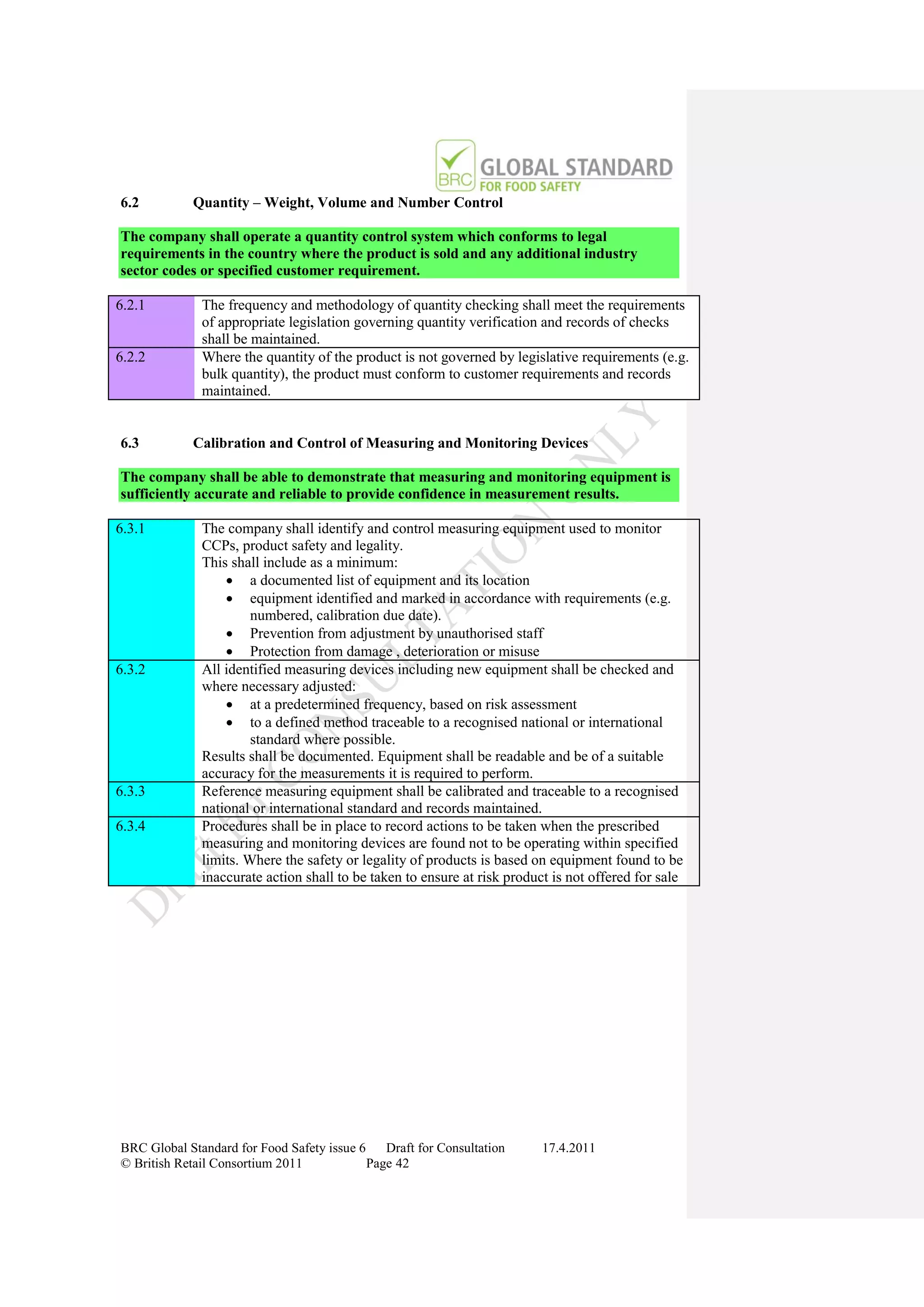
The document is a draft of the sixth edition of the BRC Global Standard for Food Safety. It outlines proposed changes to the standard including increasing the focus on good manufacturing practices during audits, refreshing some requirements for clarification, and introducing options for unannounced audits. Feedback is sought on the draft standard by May 18, 2011.



















![Figure 2 Comment [MS1]: Note to typesetter: do
not insert Figure 2 in the middle of bulleted
list.
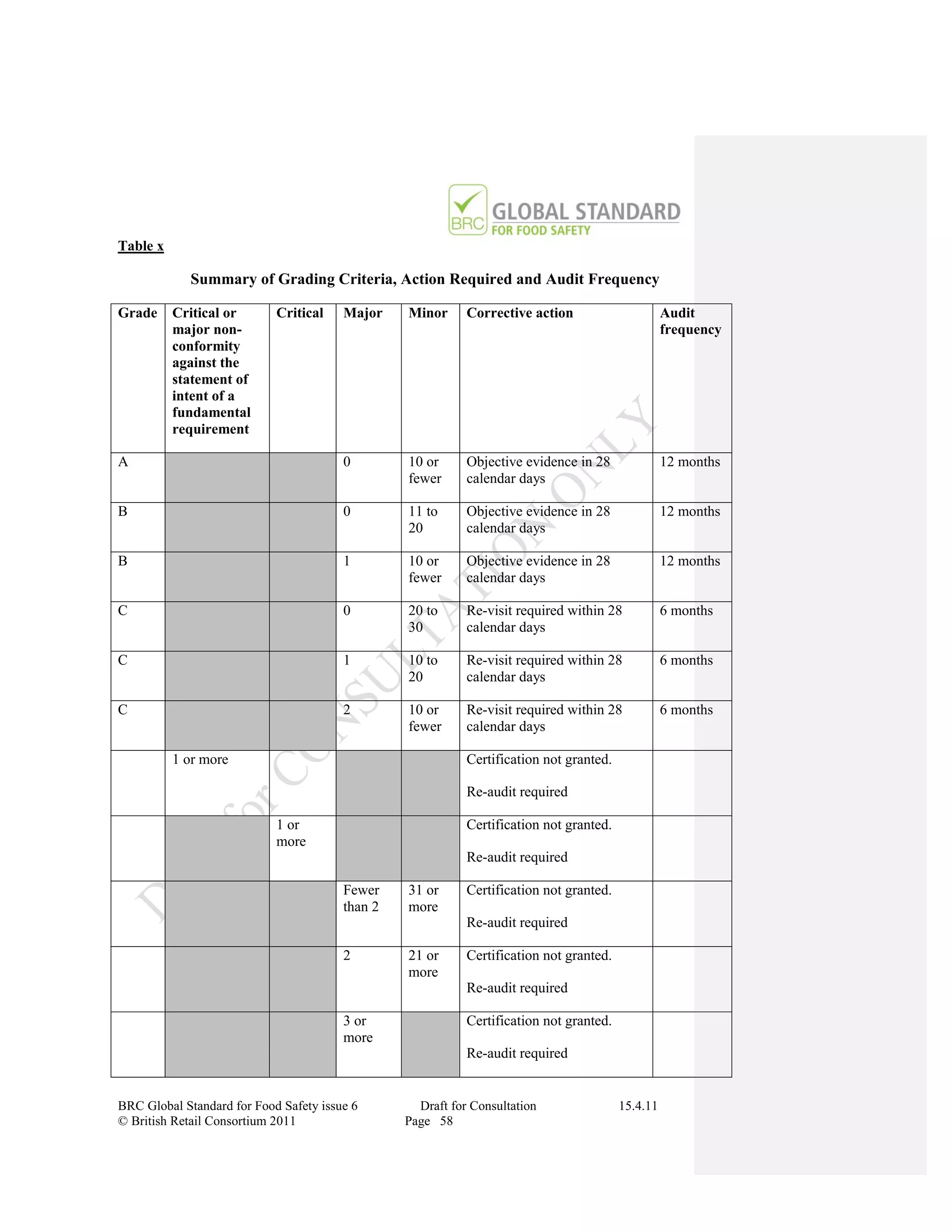
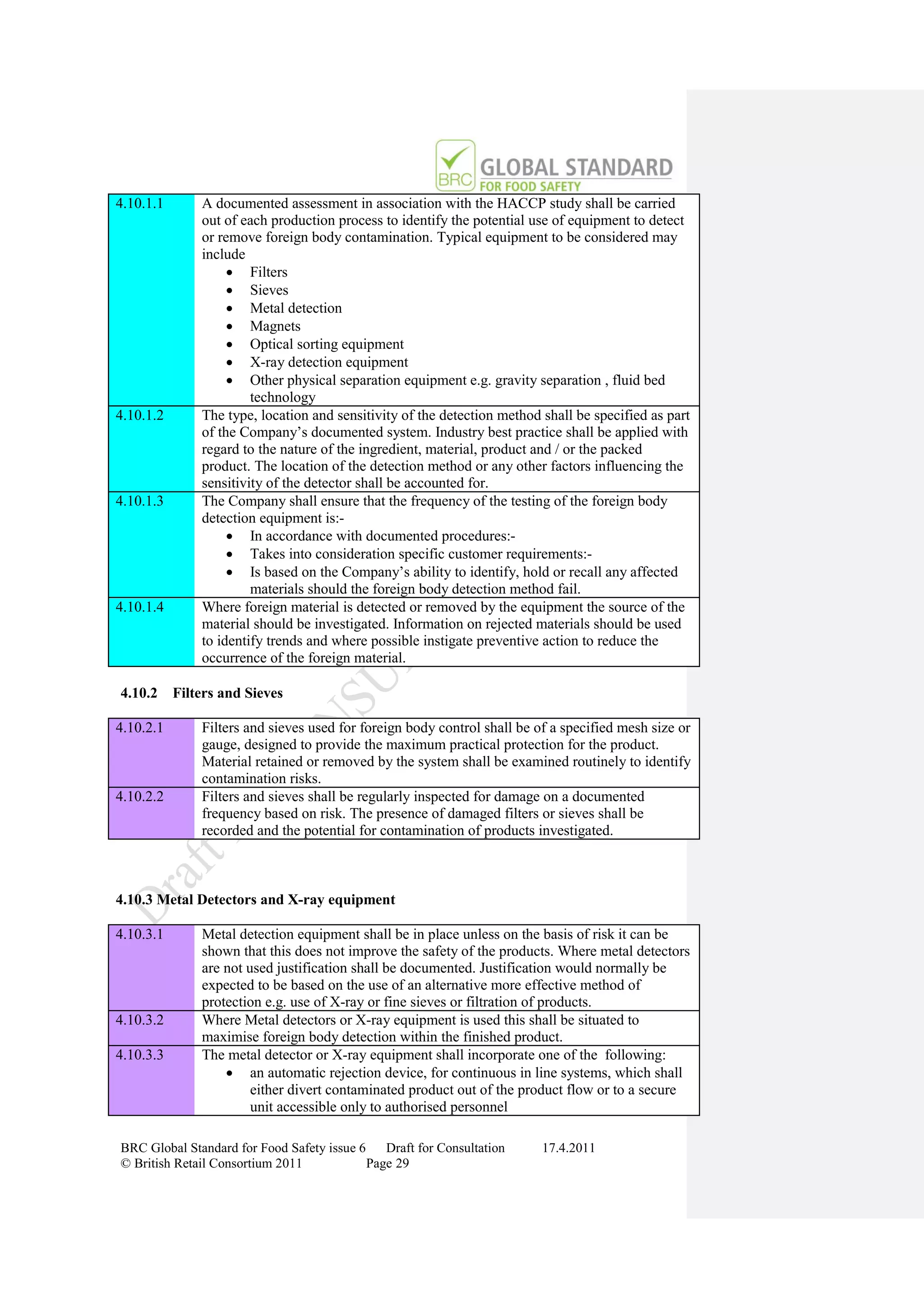
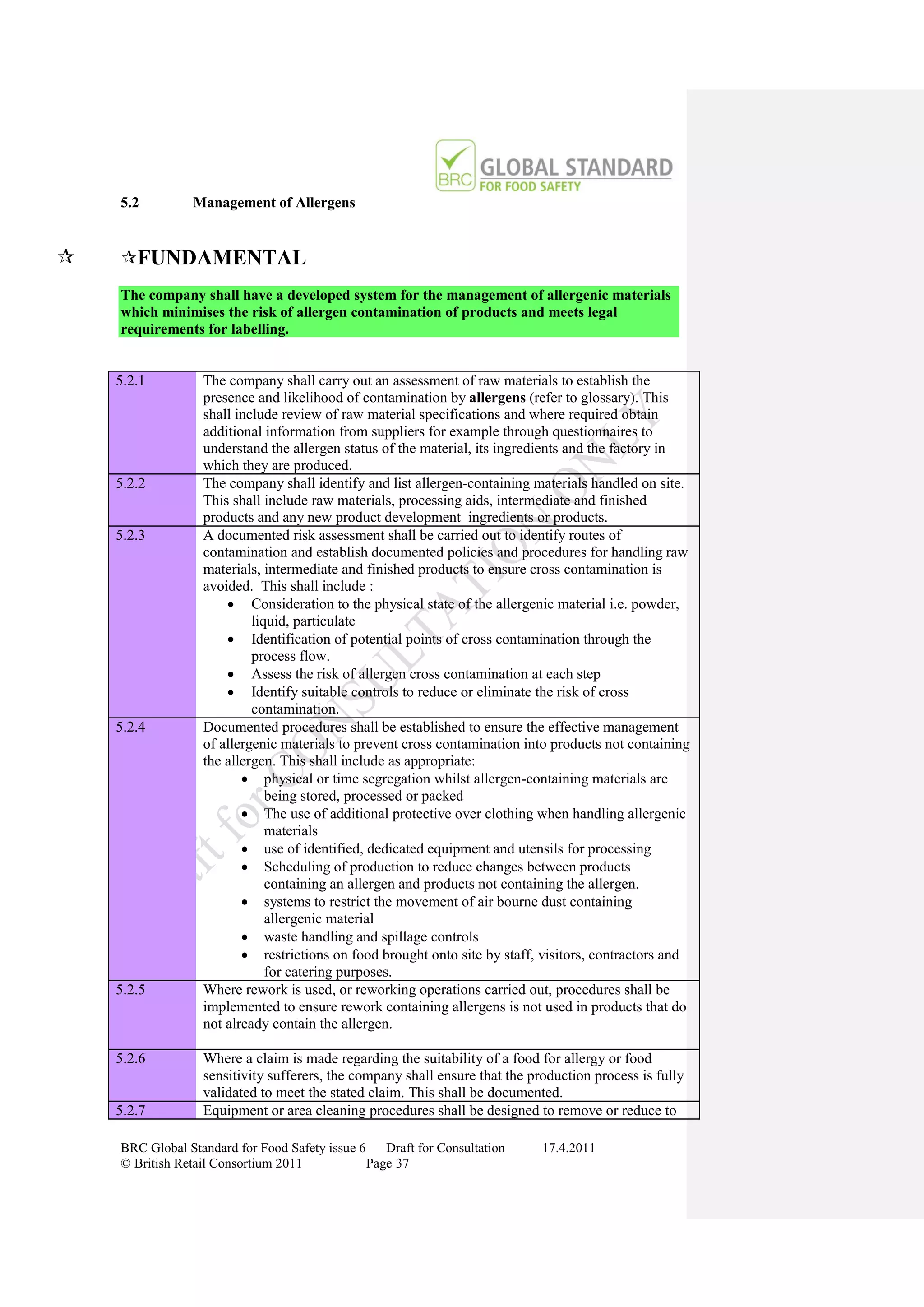
How to Gain Certification
Obtain Copy Of Standard
www.brcglobalstandards.com
Self Assessment Compliance
With Standard
Select Certification Body
www.brcdirectory.com
Pre-assessment (Optional)
On Site Audit
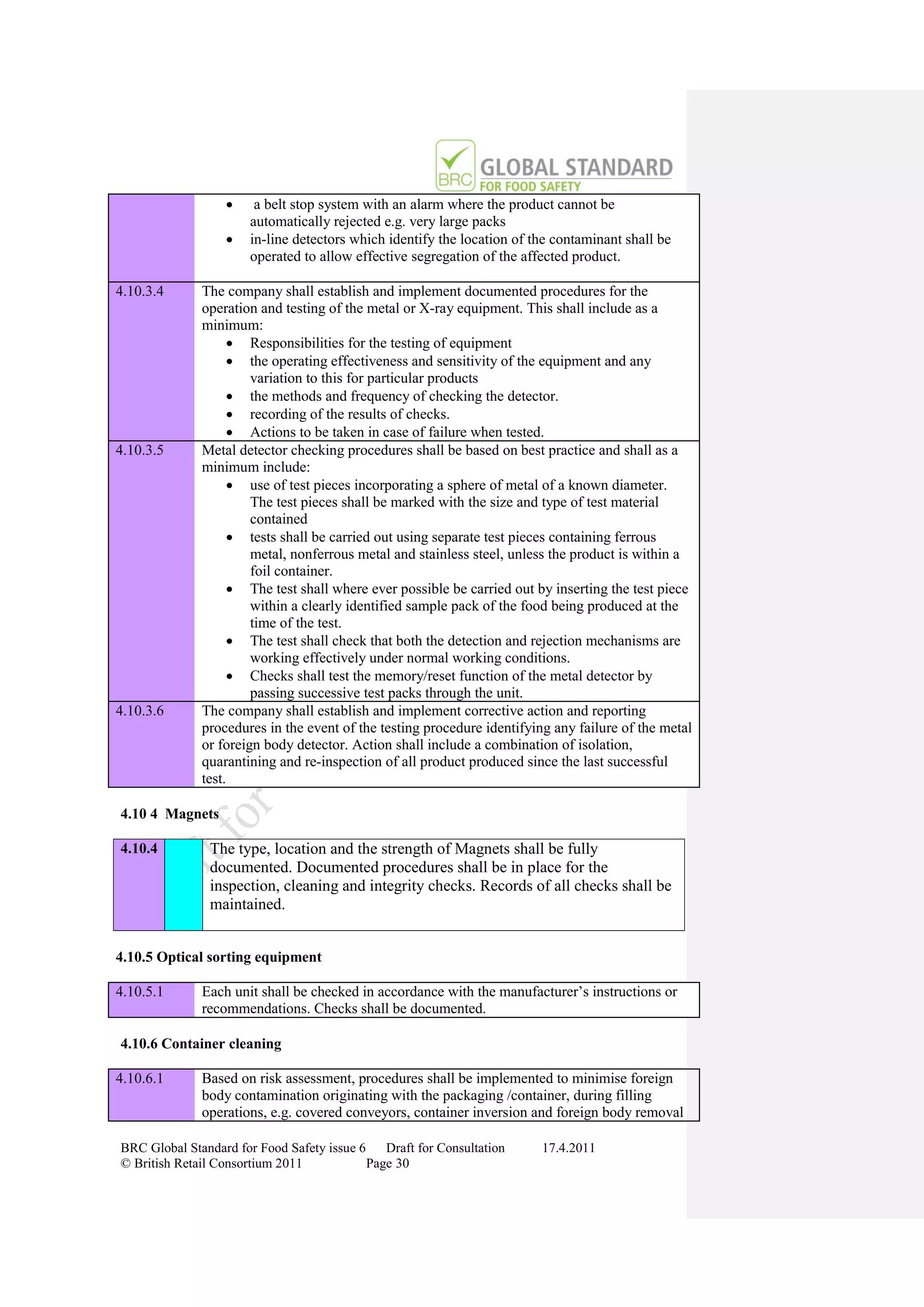
Closing Meeting Critical or Major Non-
Confirmation of any Non- conformity against
conformities Fundamental Requirement
Corrective Action
Submitted or Revisit No Non-conformities
Within 28 Calendar Identified
Days
No Corrective Action
Evidence Assessed Submitted Within 28 Days
Evidence Assessed Clarification
Inadequate Required
Additional Evidence Assessed
No Certificate Issued
Clarification Compliant
Report Issued
Provided
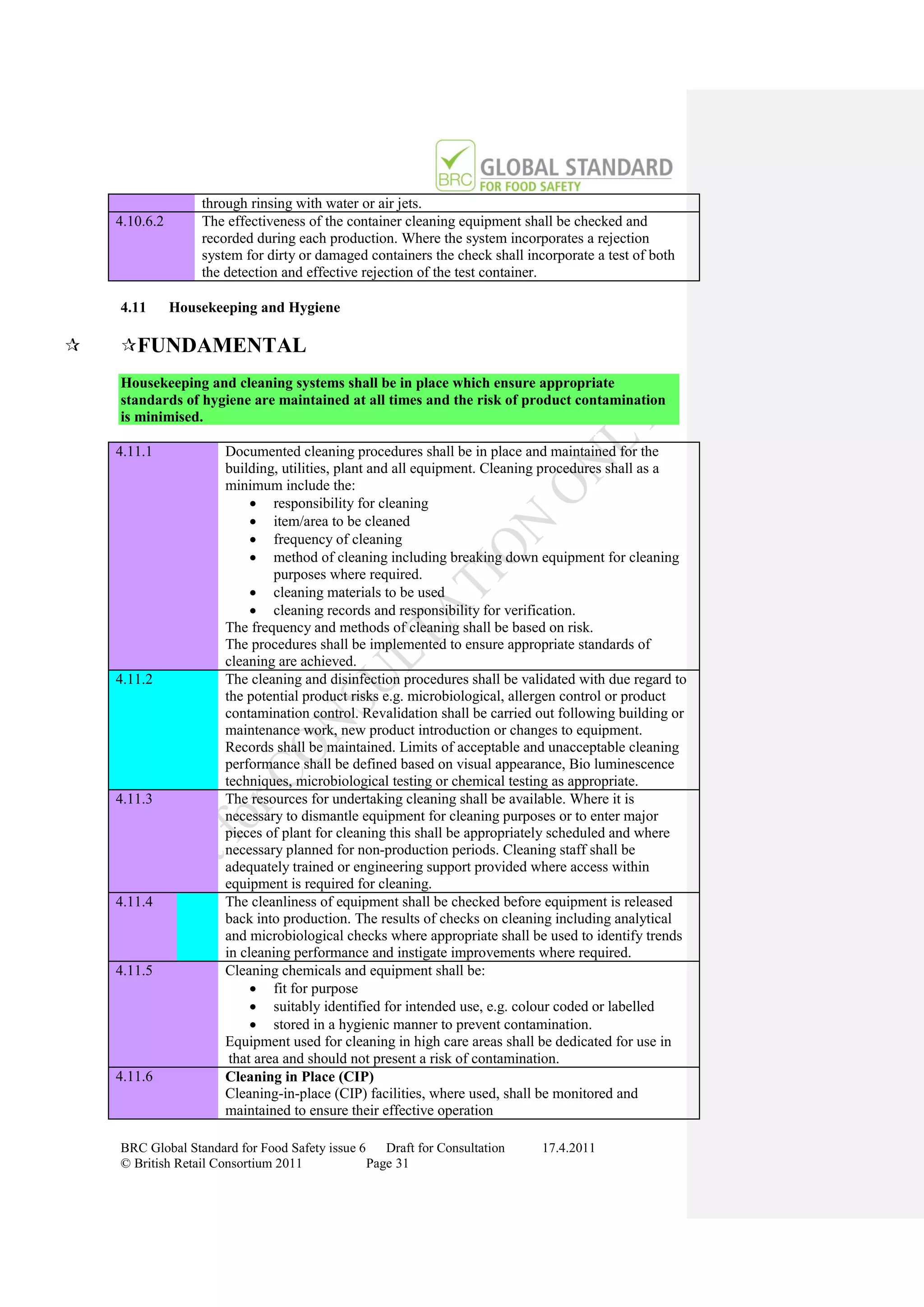
Specifying Status
Process / Certification
Suspended
Corrective Action
Status Documented
Certification
Documentation
Collated
No Certificate
Issued Certification and
Report Issued Evidence Grading Decision
Specifying Status Assessed made by Certification
Process/ Inadequate Decision Manager
Certification
Suspended
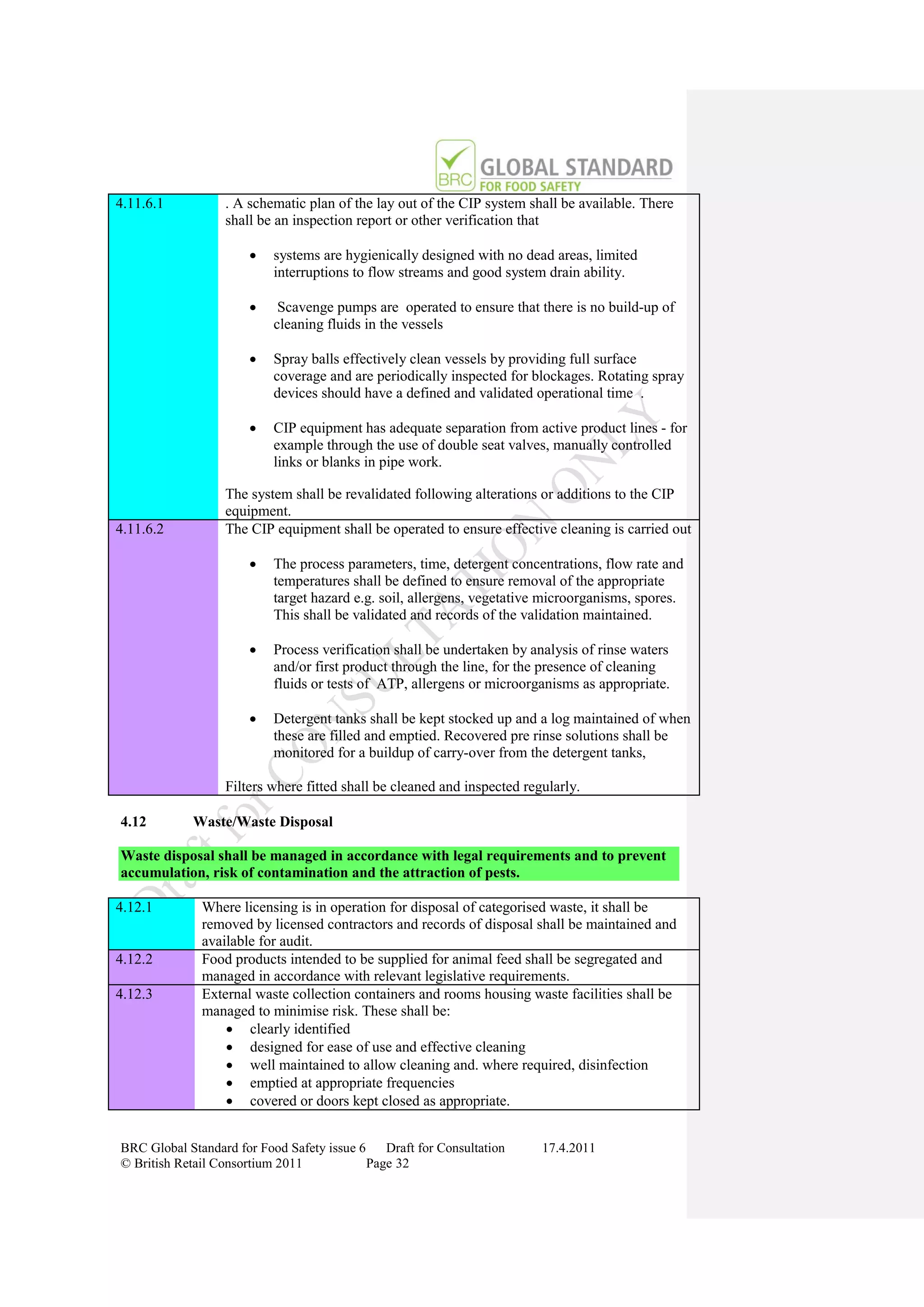
Certificate Details and Audit Report
Issued to Company and sent to BRC
Critical or Major
(Fundamental)
On Going Non-conformity
Certificate and Audit Details listed Identified
on BRC Global Standards Directory Compliance
Certification
www.brcdirectory.com Suspended
Audit
In Accordance With
Required Frequency
BRC Global Standard for Food Safety issue 6 Draft for Consultation 17.4.2011
© British Retail Consortium 2011 Page 50](https://image.slidesharecdn.com/74c28e8f76-110602035119-phpapp02/75/BRC-6-Taslak-50-2048.jpg)